Does Pressure Affect Rate Of Diffusion . Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. The rate of diffusion in this instance is almost totally dependent on pressure. Faster movement equals faster diffusion. Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The factors affecting rate of diffusion are: Diffusion occurs when particles move from an area of high concentration to an area of low concentration. They state that ‘the rate of diffusion is directly proportional to both the. The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. Effusion refers to the movement of gas. Effect of pressure on diffusion: One of the effects of high blood pressure is the appearance of protein in the. Hence the particles of the different. The different factors that affect diffusion either individually or collectively are: To a rough approximation, gases diffuse about 100,000 times faster than do liquids.
from www.slideserve.com
Faster movement equals faster diffusion. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. Warmer the temperature, higher is the rate of diffusion. Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. They state that ‘the rate of diffusion is directly proportional to both the. When the pressure increases then the particles comes close to each other. The factors affecting rate of diffusion are:
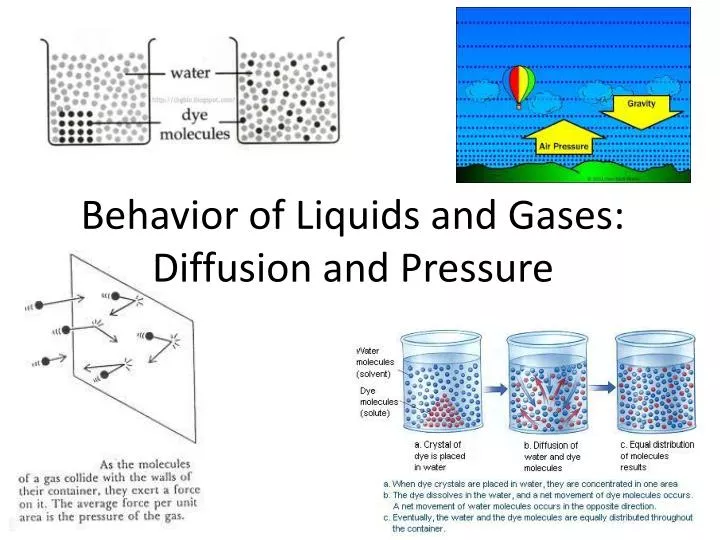
PPT Behavior of Liquids and Gases Diffusion and Pressure PowerPoint Presentation ID3032237
Does Pressure Affect Rate Of Diffusion Warmer the temperature, higher is the rate of diffusion. Effusion refers to the movement of gas. The rate of diffusion in this instance is almost totally dependent on pressure. The different factors that affect diffusion either individually or collectively are: Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. Hence the particles of the different. The factors affecting rate of diffusion are: Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. They state that ‘the rate of diffusion is directly proportional to both the. Faster movement equals faster diffusion. The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. When the pressure increases then the particles comes close to each other. Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Effect of pressure on diffusion: Warmer the temperature, higher is the rate of diffusion. To a rough approximation, gases diffuse about 100,000 times faster than do liquids.
From www.slideshare.net
Lesson 13 diffusion Does Pressure Affect Rate Of Diffusion To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Effect of pressure on diffusion: One of the effects of high blood pressure is the appearance of protein in the. Diffusion occurs when particles move from an area of high concentration to an area of low concentration. Diffusion is faster at higher temperatures because the gas molecules. Does Pressure Affect Rate Of Diffusion.
From lessonlistkettlefuls.z13.web.core.windows.net
How Does Temperature Affect Diffusion Does Pressure Affect Rate Of Diffusion The rate of diffusion in this instance is almost totally dependent on pressure. The factors affecting rate of diffusion are: Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. The different factors that affect diffusion either individually. Does Pressure Affect Rate Of Diffusion.
From www.sciencefacts.net
Fick’s Laws of Diffusion Formulas, Equations, & Examples Does Pressure Affect Rate Of Diffusion Warmer the temperature, higher is the rate of diffusion. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. The rate of diffusion in this instance is almost totally dependent on pressure. Concentration, temperature, mass of the particle and properties of the solvent. Does Pressure Affect Rate Of Diffusion.
From www.youtube.com
Factors Affecting The Rate of Diffusion Across Cellular Membranes YouTube Does Pressure Affect Rate Of Diffusion They state that ‘the rate of diffusion is directly proportional to both the. The different factors that affect diffusion either individually or collectively are: Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Diffusion occurs when particles move from an area of high concentration to an area of low concentration. The rate of diffusion. Does Pressure Affect Rate Of Diffusion.
From quizlet.com
Factors Affecting the Rate of Diffusion Diagram Quizlet Does Pressure Affect Rate Of Diffusion One of the effects of high blood pressure is the appearance of protein in the. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The factors affecting rate of diffusion are: Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. To a rough approximation, gases diffuse about 100,000 times. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Cell Structure and Function PowerPoint Presentation, free download ID3143365 Does Pressure Affect Rate Of Diffusion They state that ‘the rate of diffusion is directly proportional to both the. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. When the pressure increases then the particles comes close to each other. Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Increasing pressure can increase the rate of. Does Pressure Affect Rate Of Diffusion.
From www.youtube.com
The Effect of Temperature on Diffusion Rate Understand the Factors That Affect Diffusion Does Pressure Affect Rate Of Diffusion Faster movement equals faster diffusion. Hence the particles of the different. The factors affecting rate of diffusion are: They state that ‘the rate of diffusion is directly proportional to both the. Warmer the temperature, higher is the rate of diffusion. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The rate of diffusion in this. Does Pressure Affect Rate Of Diffusion.
From www.expii.com
Diffusion and Effusion — Definition & Overview Expii Does Pressure Affect Rate Of Diffusion When the pressure increases then the particles comes close to each other. Hence the particles of the different. One of the effects of high blood pressure is the appearance of protein in the. Faster movement equals faster diffusion. Diffusion occurs when particles move from an area of high concentration to an area of low concentration. Increasing pressure can increase the. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT MOVEMENT OF SUBSTANCES ACROSS THE PLASMA MEMBRANE PowerPoint Presentation ID1183138 Does Pressure Affect Rate Of Diffusion Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. Faster movement equals faster diffusion. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Effect of pressure on diffusion: Warmer the temperature, higher is the rate of. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT TRANSPORT ACROSS CELL MEMBRANE1 (Guyton, 12 th Ed. (chapter 4) pg 4556) PowerPoint Does Pressure Affect Rate Of Diffusion To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Diffusion occurs when particles move from an area of high concentration to an area of low concentration. Hence the particles of the different. Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Faster movement equals faster diffusion. Warmer the temperature, higher. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID257643 Does Pressure Affect Rate Of Diffusion One of the effects of high blood pressure is the appearance of protein in the. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. When the pressure increases then the particles comes close to each other. The different factors that affect diffusion either individually or collectively are: The factors affecting rate of diffusion are: Effect. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Diffusion (continued) PowerPoint Presentation, free download ID1534396 Does Pressure Affect Rate Of Diffusion They state that ‘the rate of diffusion is directly proportional to both the. The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Warmer the temperature, higher is the rate of diffusion. To a rough approximation, gases diffuse. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Membrane Transport PowerPoint Presentation, free download ID438880 Does Pressure Affect Rate Of Diffusion One of the effects of high blood pressure is the appearance of protein in the. They state that ‘the rate of diffusion is directly proportional to both the. When the pressure increases then the particles comes close to each other. The factors affecting rate of diffusion are: Effect of pressure on diffusion: Faster movement equals faster diffusion. Warmer the temperature,. Does Pressure Affect Rate Of Diffusion.
From eduinput.com
Diffusion Explained Types, Examples and Factors Does Pressure Affect Rate Of Diffusion Effect of pressure on diffusion: Faster movement equals faster diffusion. Hence the particles of the different. The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. Warmer the temperature, higher is the rate of diffusion. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Increasing pressure can. Does Pressure Affect Rate Of Diffusion.
From www.youtube.com
Factors that Affect Diffusion Rate YouTube Does Pressure Affect Rate Of Diffusion The different factors that affect diffusion either individually or collectively are: Effusion refers to the movement of gas. One of the effects of high blood pressure is the appearance of protein in the. Hence the particles of the different. The factors affecting rate of diffusion are: Faster movement equals faster diffusion. Increasing pressure can increase the rate of diffusion, while. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Graham’s Law of Diffusion PowerPoint Presentation, free download ID4693447 Does Pressure Affect Rate Of Diffusion To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Warmer the temperature, higher is the rate of diffusion. The different factors that affect diffusion either individually or collectively are: Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Effusion refers to the movement of gas. The rate of diffusion in. Does Pressure Affect Rate Of Diffusion.
From www.mooramo.com
Factors That Affect the Rate of Diffusion Mooramo Does Pressure Affect Rate Of Diffusion Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Effusion refers to the movement of gas. The rate of diffusion in this instance is almost totally dependent on pressure. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Concentration, temperature, mass of the particle and properties of the solvent in which. Does Pressure Affect Rate Of Diffusion.
From thescienceteacher.co.uk
Diffusion teaching resources the science teacher Does Pressure Affect Rate Of Diffusion Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. They state that ‘the rate of diffusion is directly proportional to both the. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Faster movement equals faster diffusion. Warmer the temperature, higher is the rate of diffusion. One of the effects of high. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Diffusion, Osmosis and Osmotic Pressure PowerPoint Presentation, free download ID7003986 Does Pressure Affect Rate Of Diffusion When the pressure increases then the particles comes close to each other. Diffusion occurs when particles move from an area of high concentration to an area of low concentration. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Faster movement equals faster diffusion. Concentration, temperature, mass of the particle and properties of the solvent in which. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID5845580 Does Pressure Affect Rate Of Diffusion Warmer the temperature, higher is the rate of diffusion. The different factors that affect diffusion either individually or collectively are: Effect of pressure on diffusion: When the pressure increases then the particles comes close to each other. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Faster movement equals faster diffusion. To a rough approximation,. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Passive and Active Transport PowerPoint Presentation ID2484371 Does Pressure Affect Rate Of Diffusion Warmer the temperature, higher is the rate of diffusion. Diffusion occurs when particles move from an area of high concentration to an area of low concentration. One of the effects of high blood pressure is the appearance of protein in the. Hence the particles of the different. The laws also describe the relationship between the rate of diffusion and the. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT The Cell Membrane PowerPoint Presentation, free download ID2927222 Does Pressure Affect Rate Of Diffusion The different factors that affect diffusion either individually or collectively are: Diffusion occurs when particles move from an area of high concentration to an area of low concentration. Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. Warmer the temperature, higher is the rate of diffusion. Effusion refers to the movement of gas. Hence the. Does Pressure Affect Rate Of Diffusion.
From www.sliderbase.com
Crossing Membranes Passive Processes Presentation Cell biology Does Pressure Affect Rate Of Diffusion Faster movement equals faster diffusion. The different factors that affect diffusion either individually or collectively are: One of the effects of high blood pressure is the appearance of protein in the. Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. The factors affecting rate of diffusion are: They state that ‘the rate of diffusion. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Behavior of Liquids and Gases Diffusion and Pressure PowerPoint Presentation ID3032237 Does Pressure Affect Rate Of Diffusion Diffusion occurs when particles move from an area of high concentration to an area of low concentration. The rate of diffusion in this instance is almost totally dependent on pressure. Faster movement equals faster diffusion. Effect of pressure on diffusion: Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The different factors that affect diffusion. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2151928 Does Pressure Affect Rate Of Diffusion The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. Hence the particles of the different. Diffusion occurs when particles move from an area of high concentration to an area of low concentration. The rate of diffusion in this instance is almost totally dependent on pressure. Warmer the temperature, higher is the. Does Pressure Affect Rate Of Diffusion.
From studymind.co.uk
Transport Across Membranes Diffusion (Alevel Biology) Study Mind Does Pressure Affect Rate Of Diffusion Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. When the pressure increases then the particles comes close to each other. Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. Effusion refers to the movement of gas. One of the effects of high blood pressure is the appearance of. Does Pressure Affect Rate Of Diffusion.
From derangedphysiology.com
Diffusion of gases through the alveolar membrane Deranged Physiology Does Pressure Affect Rate Of Diffusion The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. Hence the particles of the different. The different factors that affect diffusion either individually or collectively are: Effect of pressure on diffusion: The factors affecting rate of diffusion are: They state that ‘the rate of diffusion is directly proportional to both the.. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Diffusion, Osmosis and Osmotic Pressure PowerPoint Presentation, free download ID7003986 Does Pressure Affect Rate Of Diffusion They state that ‘the rate of diffusion is directly proportional to both the. When the pressure increases then the particles comes close to each other. The rate of diffusion in this instance is almost totally dependent on pressure. Effect of pressure on diffusion: The laws also describe the relationship between the rate of diffusion and the three factors that affect. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT The Cell Membrane PowerPoint Presentation ID7068362 Does Pressure Affect Rate Of Diffusion Effect of pressure on diffusion: The factors affecting rate of diffusion are: Hence the particles of the different. They state that ‘the rate of diffusion is directly proportional to both the. Effusion refers to the movement of gas. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. Faster movement equals faster diffusion. Diffusion is faster at. Does Pressure Affect Rate Of Diffusion.
From mavink.com
Rate Of Diffusion Graph Does Pressure Affect Rate Of Diffusion Hence the particles of the different. Faster movement equals faster diffusion. When the pressure increases then the particles comes close to each other. Effect of pressure on diffusion: The factors affecting rate of diffusion are: Diffusion occurs when particles move from an area of high concentration to an area of low concentration. To a rough approximation, gases diffuse about 100,000. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Theory and Gases PowerPoint Presentation, free download ID513631 Does Pressure Affect Rate Of Diffusion Faster movement equals faster diffusion. One of the effects of high blood pressure is the appearance of protein in the. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The rate of diffusion in this instance is almost totally dependent on pressure. The different factors that affect diffusion either individually or collectively are: The laws. Does Pressure Affect Rate Of Diffusion.
From www.savemyexams.co.uk
Factors Affecting the Rate of Diffusion (8.1.2) Edexcel GCSE Biology Revision Notes 2018 Does Pressure Affect Rate Of Diffusion The rate of diffusion in this instance is almost totally dependent on pressure. They state that ‘the rate of diffusion is directly proportional to both the. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Faster movement equals faster diffusion. Warmer the temperature, higher is the rate of diffusion. Diffusion occurs when particles move from. Does Pressure Affect Rate Of Diffusion.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6716640 Does Pressure Affect Rate Of Diffusion Increasing pressure can increase the rate of diffusion, while decreasing pressure can decrease it. The different factors that affect diffusion either individually or collectively are: Warmer the temperature, higher is the rate of diffusion. Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Diffusion is faster at higher temperatures because the gas molecules have. Does Pressure Affect Rate Of Diffusion.
From www.researchgate.net
The effect of concentration of a substance on the rate of diffusion... Download Scientific Diagram Does Pressure Affect Rate Of Diffusion They state that ‘the rate of diffusion is directly proportional to both the. Warmer the temperature, higher is the rate of diffusion. Concentration, temperature, mass of the particle and properties of the solvent in which diffusion occurs. Hence the particles of the different. The different factors that affect diffusion either individually or collectively are: The laws also describe the relationship. Does Pressure Affect Rate Of Diffusion.
From byjus.com
Give a simple activity/experiment to show that the rate of diffusion increases with rising Does Pressure Affect Rate Of Diffusion When the pressure increases then the particles comes close to each other. To a rough approximation, gases diffuse about 100,000 times faster than do liquids. The factors affecting rate of diffusion are: Effusion refers to the movement of gas. The laws also describe the relationship between the rate of diffusion and the three factors that affect diffusion. Increasing pressure can. Does Pressure Affect Rate Of Diffusion.