Fda Microbial Limits For Cosmetics . Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Does the presence of probiotics in cosmetics affect the shelf life. Fda is looking closely at the microbiological safety of cosmetics. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Not detected using a method that can detect one colony forming unit (cfu) per 5.
from www.mdpi.com
The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Fda is looking closely at the microbiological safety of cosmetics. Does the presence of probiotics in cosmetics affect the shelf life. Not detected using a method that can detect one colony forming unit (cfu) per 5. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics.
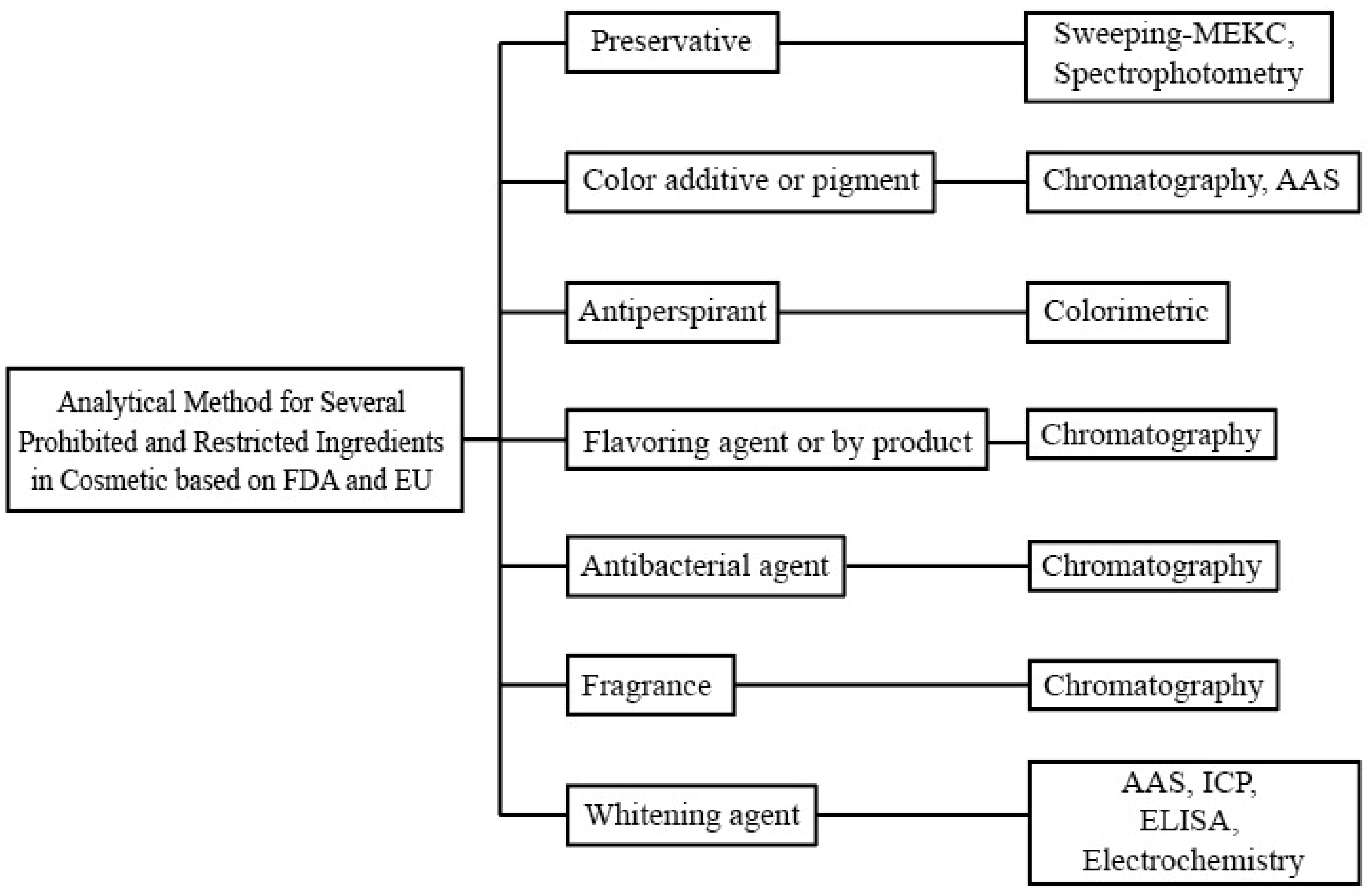
Cosmetics Free FullText Analysis of Prohibited and Restricted Ingredients in Cosmetics HTML
Fda Microbial Limits For Cosmetics Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Fda is looking closely at the microbiological safety of cosmetics. Not detected using a method that can detect one colony forming unit (cfu) per 5. Does the presence of probiotics in cosmetics affect the shelf life. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury.
From www.mdpi.com
Cosmetics Free FullText Analysis of Prohibited and Restricted Ingredients in Cosmetics HTML Fda Microbial Limits For Cosmetics Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Does the presence of probiotics in cosmetics affect the shelf life. How can cosmetics containing live microorganisms meet existing microbial contamination limits? The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Fda is looking closely at the microbiological safety of. Fda Microbial Limits For Cosmetics.
From www.scribd.com
ISO 17516 A New Standard For Microbiological Limits in Cosmetics PDF Fda Microbial Limits For Cosmetics Fda is looking closely at the microbiological safety of cosmetics. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Not detected using a method that can detect one colony forming unit (cfu) per 5. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Does the presence of probiotics in cosmetics affect the shelf life. The. Fda Microbial Limits For Cosmetics.
From www.researchgate.net
microbial limits for botanical ingredients. Download Table Fda Microbial Limits For Cosmetics Not detected using a method that can detect one colony forming unit (cfu) per 5. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Fda is looking closely at the microbiological safety of cosmetics. Does the presence of probiotics in cosmetics affect the shelf life. Cosmetics can become harmful to consumers if. Fda Microbial Limits For Cosmetics.
From www.semanticscholar.org
Guidelines for the microbiological quality of some readytoeat foods sampled at the point of Fda Microbial Limits For Cosmetics Not detected using a method that can detect one colony forming unit (cfu) per 5. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Fda is looking closely at the microbiological safety of cosmetics. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory. Fda Microbial Limits For Cosmetics.
From pharmablog.in
Microbial Limit Test for Finished Products SOP PharmaBlog Fda Microbial Limits For Cosmetics Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Fda is looking closely at the microbiological safety of cosmetics. Does the presence of. Fda Microbial Limits For Cosmetics.
From www.bioscontrol-safecare.com
Cosmetics microbial safety a milestone BiosControl Fda Microbial Limits For Cosmetics Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Fda is looking closely at the microbiological safety of cosmetics. Not detected using a method that can detect one colony forming unit (cfu) per 5. The ability of microorganisms to. Fda Microbial Limits For Cosmetics.
From www.nutraceuticalsworld.com
Understanding & Mitigating Microbial Contaminants In Herbal Dietary Supplements Nutraceuticals Fda Microbial Limits For Cosmetics Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and.. Fda Microbial Limits For Cosmetics.
From www.cosmeticsandtoiletries.com
Determining Preservative Efficacy in W/O Formulas, Part II Microbiological Safety Assessment Fda Microbial Limits For Cosmetics Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and. Fda Microbial Limits For Cosmetics.
From www.en-standard.eu
BS EN ISO 175162014 Cosmetics. Microbiology. Microbiological limits Fda Microbial Limits For Cosmetics Does the presence of probiotics in cosmetics affect the shelf life. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Table 1 describes. Fda Microbial Limits For Cosmetics.
From www.researchgate.net
Microbial count results and permitted limits according to the FDA¹⁴ and... Download Scientific Fda Microbial Limits For Cosmetics The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Does the presence of probiotics in cosmetics affect the shelf life. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. How can cosmetics containing live microorganisms. Fda Microbial Limits For Cosmetics.
From www.scribd.com
Microbial Limit Test Validation Protocol PDF Growth Medium Microbiology Fda Microbial Limits For Cosmetics Fda is looking closely at the microbiological safety of cosmetics. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Does the presence of probiotics in cosmetics affect the shelf life. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such. Fda Microbial Limits For Cosmetics.
From www.researchgate.net
Existing microbiological limits and guidelines for Vibrio... Download Table Fda Microbial Limits For Cosmetics Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Fda is looking closely at the microbiological safety of cosmetics. Not detected using a method that can detect one colony forming unit (cfu) per 5. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Microorganisms may cause. Fda Microbial Limits For Cosmetics.
From www.slideshare.net
Microbial limit test Fda Microbial Limits For Cosmetics The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Does the presence of probiotics in cosmetics affect the shelf life. Microorganisms may cause spoilage or chemical changes. Fda Microbial Limits For Cosmetics.
From www.youtube.com
Difference Between BIOBURDEN TEST AND MICROBIAL LIMIT TEST YouTube Fda Microbial Limits For Cosmetics Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Does the presence of probiotics in cosmetics affect the shelf life. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Microorganisms may cause spoilage or chemical changes. Fda Microbial Limits For Cosmetics.
From www.slideshare.net
Microbial limit test Fda Microbial Limits For Cosmetics Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Not detected using a method that can detect one colony forming unit (cfu) per 5. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. How. Fda Microbial Limits For Cosmetics.
From meredithteutro.blogspot.com
Fda Coliform Limit Fda Microbial Limits For Cosmetics Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Does the presence of probiotics in cosmetics affect the shelf life. Not detected using a method that can detect one colony forming unit (cfu) per 5. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Fda is looking. Fda Microbial Limits For Cosmetics.
From ethidelabs.com
Ethide Laboratories Bacterial Endotoxin Limits And Calculations For Drugs And Biologics Fda Microbial Limits For Cosmetics Not detected using a method that can detect one colony forming unit (cfu) per 5. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Fda is looking closely at the microbiological safety of cosmetics. Does the presence of probiotics in cosmetics affect the shelf life. The ability of microorganisms to grow and. Fda Microbial Limits For Cosmetics.
From resorcio.com
Microbial Limit Test Fda Microbial Limits For Cosmetics Does the presence of probiotics in cosmetics affect the shelf life. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. The ability of microorganisms to grow and reproduce. Fda Microbial Limits For Cosmetics.
From www.researchgate.net
(PDF) Microbial Quality Assurance and Quality Control in Cosmetics Fda Microbial Limits For Cosmetics The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Fda is looking closely at the microbiological safety of cosmetics. How can. Fda Microbial Limits For Cosmetics.
From www.slideshare.net
Iso 17516 2014 cosmetics microbiological limits PDF Fda Microbial Limits For Cosmetics Fda is looking closely at the microbiological safety of cosmetics. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Not detected using a method that can detect one colony forming unit (cfu) per 5. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. The ability of microorganisms to grow. Fda Microbial Limits For Cosmetics.
From microbiologyguideritesh.com
Flow Chart of Microbial Limit Test Microbiology Guidelines Fda Microbial Limits For Cosmetics Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Fda is looking closely at the microbiological safety of cosmetics. Table 1 describes general. Fda Microbial Limits For Cosmetics.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Fda Microbial Limits For Cosmetics Fda is looking closely at the microbiological safety of cosmetics. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Table 1 describes general guidelines for qualitative methods to detect. Fda Microbial Limits For Cosmetics.
From www.researchgate.net
Microbiological Limits for Assessment of Microbiological Quality Download Table Fda Microbial Limits For Cosmetics Does the presence of probiotics in cosmetics affect the shelf life. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Not detected using a method that can detect one colony forming unit (cfu) per 5. Fda's bacteriological analytical manual (bam) presents the agency's. Fda Microbial Limits For Cosmetics.
From www.fda.gov.ph
FDA Circular Food and Drug Administration Fda Microbial Limits For Cosmetics Does the presence of probiotics in cosmetics affect the shelf life. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. The ability of microorganisms to grow and. Fda Microbial Limits For Cosmetics.
From www.slideserve.com
PPT What Is Microbial Limit Test? PowerPoint Presentation, free download ID10938861 Fda Microbial Limits For Cosmetics Fda is looking closely at the microbiological safety of cosmetics. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Cosmetics. Fda Microbial Limits For Cosmetics.
From cptclabs.com
Reliable Microbial Limits Test CPT Labs Fda Microbial Limits For Cosmetics Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for. Fda Microbial Limits For Cosmetics.
From www.pharmamanufacturing.com
Pharmaceutical USP Regulations USP Updates and for Microbial Testing of NonSteriles Fda Microbial Limits For Cosmetics Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Not detected using a method that can detect one colony forming unit (cfu) per 5. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Table 1 describes general guidelines for qualitative methods to detect. Fda Microbial Limits For Cosmetics.
From mycoscience.com
MycoScience Microbiological Testing For Nonsterile Dietary Supplements Fda Microbial Limits For Cosmetics Fda is looking closely at the microbiological safety of cosmetics. Does the presence of probiotics in cosmetics affect the shelf life. How can cosmetics containing live microorganisms meet existing microbial contamination limits? The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Table 1 describes general guidelines for qualitative methods to detect conventional. Fda Microbial Limits For Cosmetics.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Fda Microbial Limits For Cosmetics Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Does the presence of probiotics in cosmetics affect the shelf life. Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Fda is looking closely at the microbiological. Fda Microbial Limits For Cosmetics.
From www.youtube.com
Do you know the procedure of Microbial Limit Test? Microbial Analysis of Pharmaceutical Fda Microbial Limits For Cosmetics Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria. Fda Microbial Limits For Cosmetics.
From microbiologyguideritesh.com
Fundamentals of Microbial Limit Test 2023 Fda Microbial Limits For Cosmetics How can cosmetics containing live microorganisms meet existing microbial contamination limits? Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. Not detected using a method that can detect one colony forming unit (cfu) per 5. Does the presence of probiotics in cosmetics affect the shelf life. Fda is looking closely at. Fda Microbial Limits For Cosmetics.
From studylib.net
Microbial limit test Fda Microbial Limits For Cosmetics Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Fda is looking closely at the microbiological safety of cosmetics. Fda's bacteriological analytical manual (bam) presents the agency's preferred laboratory procedures for microbiological analyses of foods and cosmetics. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Cosmetics can become harmful to consumers if. Fda Microbial Limits For Cosmetics.
From www.researchgate.net
Microbial degradation of cosmetic preservatives. Download Scientific Diagram Fda Microbial Limits For Cosmetics Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. How can cosmetics containing live microorganisms meet existing microbial contamination limits? Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Fda is looking. Fda Microbial Limits For Cosmetics.
From www.presentationeze.com
Cleanroom Classification Information & current Best PracticePresentationEZE Fda Microbial Limits For Cosmetics Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. The ability of microorganisms to grow and reproduce in cosmetic products has been known for many years. Does the presence of probiotics in cosmetics affect the shelf life. Not detected using a method that can detect one colony forming unit (cfu) per 5.. Fda Microbial Limits For Cosmetics.
From www.slideshare.net
Iso 17516 2014 cosmetics microbiological limits PDF Fda Microbial Limits For Cosmetics Table 1 describes general guidelines for qualitative methods to detect conventional microbial foodborne pathogens. Fda is looking closely at the microbiological safety of cosmetics. Cosmetics can become harmful to consumers if they’re contaminated with harmful microorganisms, such as certain bacteria and. Microorganisms may cause spoilage or chemical changes in cosmetic products and injury. Does the presence of probiotics in cosmetics. Fda Microbial Limits For Cosmetics.