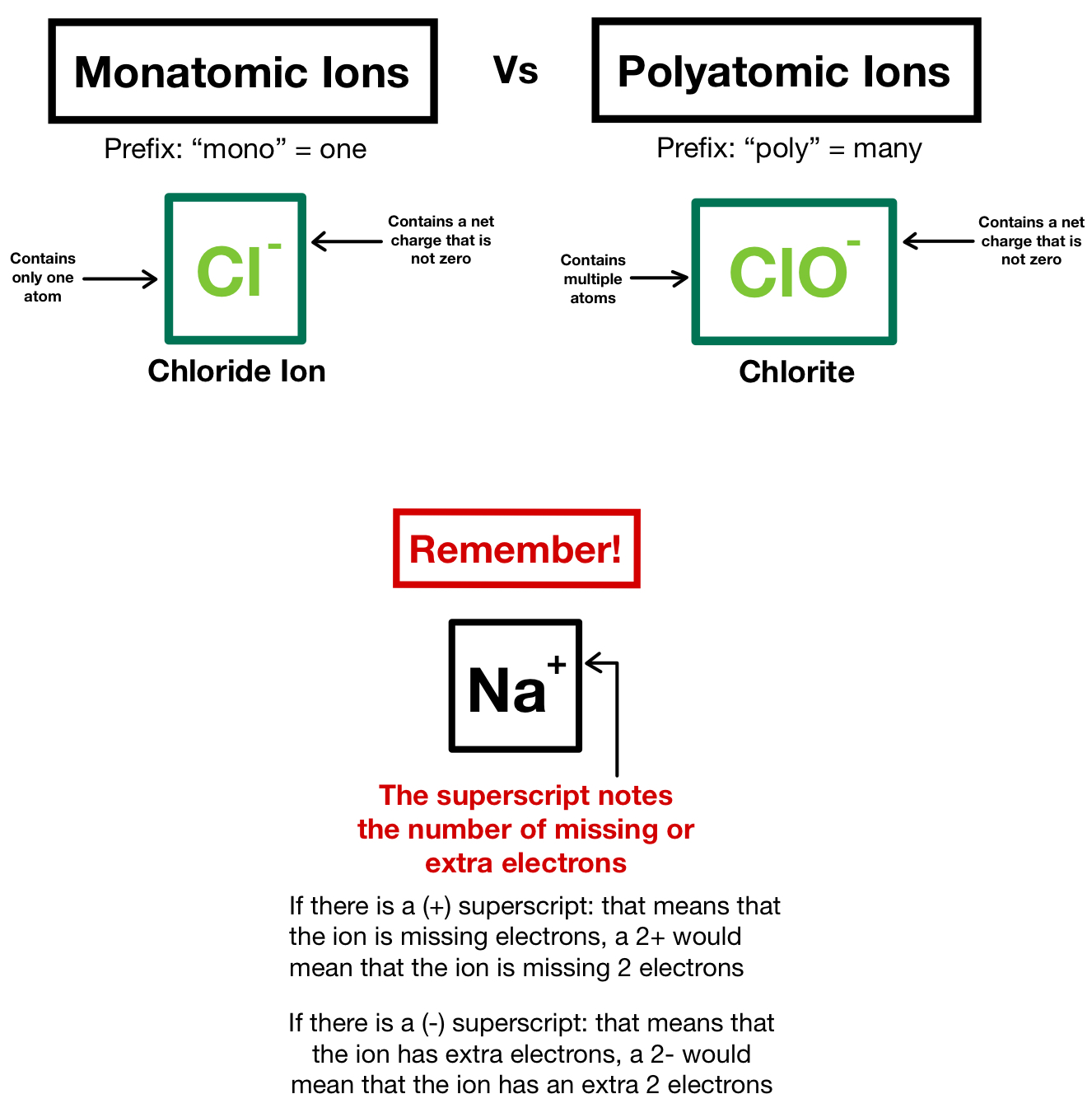
Ionic Compound Definition Biology . This type of bond forms when atoms or molecular ions. In bis2a, we focus primarily on three different bond types: Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Ionic bonds, covalent bonds, and hydrogen bonds. Ionic compounds are pure substances consisting of chemically bonded ions. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. These ions are created by the transfer of valence electrons. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic bonds are formed between ions with opposite charges. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. We expect students to be able.
from www.biologyonline.com
Ionic bonds, covalent bonds, and hydrogen bonds. Ionic bonds are formed between ions with opposite charges. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. This type of bond forms when atoms or molecular ions. In bis2a, we focus primarily on three different bond types: These ions are created by the transfer of valence electrons. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. We expect students to be able. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. Ionic compounds are pure substances consisting of chemically bonded ions.
Ion Definition and Examples Biology Online Dictionary
Ionic Compound Definition Biology In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. We expect students to be able. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. In bis2a, we focus primarily on three different bond types: Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. These ions are created by the transfer of valence electrons. This type of bond forms when atoms or molecular ions. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. Ionic bonds are formed between ions with opposite charges. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ionic bonds, covalent bonds, and hydrogen bonds. Ionic compounds are pure substances consisting of chemically bonded ions.
From ar.inspiredpencil.com
Ionic Compound Formula Ionic Compound Definition Biology We expect students to be able. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. This type of bond forms when atoms or molecular ions. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. An ionic bond is when one atom donates its. Ionic Compound Definition Biology.
From www.slideserve.com
PPT Why Study Chemistry in Biology? PowerPoint Presentation ID1957474 Ionic Compound Definition Biology An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. We expect students to be able. Ionic bonds are formed between ions with opposite charges. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Ionic bonds, covalent bonds, and hydrogen bonds.. Ionic Compound Definition Biology.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Ionic Compound Definition Biology Ionic bonds, covalent bonds, and hydrogen bonds. We expect students to be able. These ions are created by the transfer of valence electrons. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. In bis2a, we focus primarily on three different bond types: This type of bond forms when. Ionic Compound Definition Biology.
From www.chemicalindustrynews.com
Organic compound Definition and Examples Biology Online Dictionary Ionic Compound Definition Biology Ionic compounds are pure substances consisting of chemically bonded ions. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. This type of bond forms when atoms or molecular ions. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt. Ionic Compound Definition Biology.
From mungfali.com
What Are Ionic Compounds Ionic Compound Definition Biology This type of bond forms when atoms or molecular ions. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic compounds are pure substances consisting of chemically bonded ions. In the formation. Ionic Compound Definition Biology.
From sciencenotes.org
Ionic Compound Properties Ionic Compound Definition Biology Ionic bonds are formed between ions with opposite charges. These ions are created by the transfer of valence electrons. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Ionic compounds are pure substances consisting. Ionic Compound Definition Biology.
From logan-has-small.blogspot.com
What is an Ionic Bond LoganhasSmall Ionic Compound Definition Biology In bis2a, we focus primarily on three different bond types: Ionic bonds are formed between ions with opposite charges. Ionic bonds, covalent bonds, and hydrogen bonds. Ionic compounds are pure substances consisting of chemically bonded ions. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. An ionic bond is when one atom. Ionic Compound Definition Biology.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Ionic Compound Definition Biology We expect students to be able. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. This type of bond forms when atoms or molecular ions. In bis2a, we focus primarily on three different bond types: Ionic compounds are pure substances consisting of chemically bonded ions. Ionic bonds, covalent. Ionic Compound Definition Biology.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary Ionic Compound Definition Biology Ionic compounds are pure substances consisting of chemically bonded ions. This type of bond forms when atoms or molecular ions. We expect students to be able. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged. Ionic Compound Definition Biology.
From ar.inspiredpencil.com
Ionic Compound Definition Ionic Compound Definition Biology An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ionic compounds. Ionic Compound Definition Biology.
From www.differencebetween.com
Difference Between Ionic and Covalent Bonds Compare the Difference Between Similar Terms Ionic Compound Definition Biology In bis2a, we focus primarily on three different bond types: An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic bonds are formed between ions with opposite charges. This type of bond forms when atoms or molecular ions. These ions are created by the transfer of valence electrons. An. Ionic Compound Definition Biology.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Ionic Compound Definition Biology Ionic bonds, covalent bonds, and hydrogen bonds. In bis2a, we focus primarily on three different bond types: An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ionic bonds are formed between ions with opposite charges. Ionic compounds are pure substances consisting of chemically bonded ions. These ions are. Ionic Compound Definition Biology.
From www.slideserve.com
PPT Ionic Compounds Names and Formulas PowerPoint Presentation, free download ID4269213 Ionic Compound Definition Biology Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. In bis2a, we focus primarily on three different bond types: Ionic bonds, covalent bonds, and hydrogen bonds. This type of bond forms. Ionic Compound Definition Biology.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Ionic Compound Definition Biology Ionic bonds are formed between ions with opposite charges. Ionic bonds, covalent bonds, and hydrogen bonds. This type of bond forms when atoms or molecular ions. In bis2a, we focus primarily on three different bond types: In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. An atom, or group of atoms,. Ionic Compound Definition Biology.
From gamesmartz.com
Ionic Compound Definition & Image GameSmartz Ionic Compound Definition Biology These ions are created by the transfer of valence electrons. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ionic bonds are formed between ions with opposite charges. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. We expect students. Ionic Compound Definition Biology.
From ar.inspiredpencil.com
Ionic Compound Definition Ionic Compound Definition Biology Ionic compounds are pure substances consisting of chemically bonded ions. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. This type of bond forms when atoms or molecular ions. Ionic bonds are formed between ions with opposite charges. In the formation of an ionic compound, metals lose electrons and. Ionic Compound Definition Biology.
From mungfali.com
Ionic Compound Structure Ionic Compound Definition Biology In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. In bis2a, we focus primarily on three different bond types: We expect students to be able. These ions are created by the transfer of valence electrons. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein. Ionic Compound Definition Biology.
From www.animalia-life.club
Properties Of Ionic Compounds Ionic Compound Definition Biology Ionic bonds are formed between ions with opposite charges. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. We expect students to be able. Ionic bonds, covalent bonds, and hydrogen bonds. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an. Ionic Compound Definition Biology.
From learnwithdrscott.com
Ionic Bond Definition Easy Hard Science Ionic Compound Definition Biology In bis2a, we focus primarily on three different bond types: An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. This type of bond forms when atoms or molecular ions. Ionic bonds, covalent bonds, and hydrogen bonds. We expect students to be able. These ions are created by the transfer. Ionic Compound Definition Biology.
From www.slideserve.com
PPT IONIC COMPOUNDS PowerPoint Presentation, free download ID2435238 Ionic Compound Definition Biology Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. We expect students to be able. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Ionic bonds, covalent bonds, and hydrogen bonds. In bis2a, we focus primarily on three different bond. Ionic Compound Definition Biology.
From www.biologyonline.com
Ion Definition and Examples Biology Online Dictionary Ionic Compound Definition Biology In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. This type of bond forms when atoms or molecular ions. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Ionic bonds are formed between ions with opposite charges. Ionic compounds are pure substances consisting. Ionic Compound Definition Biology.
From www.slideserve.com
PPT Chemical Names of Ionic Compounds PowerPoint Presentation, free download ID1484217 Ionic Compound Definition Biology In bis2a, we focus primarily on three different bond types: An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. This type of bond forms when atoms or molecular ions. Ionic bonds, covalent bonds, and hydrogen bonds. These ions are created by the transfer of valence electrons. Ionic bonds are. Ionic Compound Definition Biology.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Ionic Compound Definition Biology These ions are created by the transfer of valence electrons. This type of bond forms when atoms or molecular ions. We expect students to be able. Ionic bonds are formed between ions with opposite charges. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. In bis2a, we focus primarily on three different. Ionic Compound Definition Biology.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download ID4435627 Ionic Compound Definition Biology An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic compounds are pure substances consisting of chemically bonded ions. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. These ions are created by the transfer of valence. Ionic Compound Definition Biology.
From www.slideserve.com
PPT CHAPTER 3 Ionic Compounds General, Organic, & Biological Chemistry Janice Gorzynski Smith Ionic Compound Definition Biology We expect students to be able. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic bonds are formed between ions with opposite charges. In bis2a, we focus primarily on three different bond types: Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions,. Ionic Compound Definition Biology.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Compound Definition Biology These ions are created by the transfer of valence electrons. Ionic compounds are pure substances consisting of chemically bonded ions. We expect students to be able. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic bonds are formed between ions with opposite charges. In bis2a, we focus primarily. Ionic Compound Definition Biology.
From sciencenotes.org
Ionic Bond Definition and Examples Ionic Compound Definition Biology In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. Ionic compounds are pure substances consisting of chemically bonded ions. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. In bis2a, we focus primarily on three different bond types: This type. Ionic Compound Definition Biology.
From ar.inspiredpencil.com
Ionic Compound Definition Ionic Compound Definition Biology Ionic compounds are pure substances consisting of chemically bonded ions. Ionic bonds, covalent bonds, and hydrogen bonds. This type of bond forms when atoms or molecular ions. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. In the formation of an ionic compound, metals lose electrons and nonmetals gain. Ionic Compound Definition Biology.
From education-portal.com
What Are Ionic Compounds? Definition, Examples & Reactions Video & Lesson Transcript Ionic Compound Definition Biology Ionic bonds are formed between ions with opposite charges. In bis2a, we focus primarily on three different bond types: Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. This type of bond forms when atoms or molecular ions. We expect students to be able. Ionic bonds, covalent bonds, and hydrogen bonds. Ionic. Ionic Compound Definition Biology.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Compound Definition Biology Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. We expect students to be able. Ionic bonds, covalent bonds, and hydrogen bonds. Ionic compounds are pure substances consisting of chemically bonded ions. In bis2a,. Ionic Compound Definition Biology.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Ionic Compound Definition Biology Ionic compounds are pure substances consisting of chemically bonded ions. Ionic bonds, covalent bonds, and hydrogen bonds. We expect students to be able. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. These ions are created by the transfer of valence electrons. This type of bond forms when. Ionic Compound Definition Biology.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free download ID6776451 Ionic Compound Definition Biology In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. This type of bond forms when atoms or molecular ions. We expect students to be able. Ionic bonds are formed between. Ionic Compound Definition Biology.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download ID6037545 Ionic Compound Definition Biology An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic bonds, covalent bonds, and hydrogen bonds. This type of bond forms when atoms or molecular ions. Ionic compounds are pure substances consisting of chemically bonded ions. These ions are created by the transfer of valence electrons. In the formation. Ionic Compound Definition Biology.
From ar.inspiredpencil.com
Ionic Compounds Examples And Their Uses Ionic Compound Definition Biology In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet. This type of bond forms when atoms or molecular ions. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. In bis2a, we focus primarily on three different bond types: We expect. Ionic Compound Definition Biology.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Compound Definition Biology In bis2a, we focus primarily on three different bond types: Ionic compounds are pure substances consisting of chemically bonded ions. An ionic bond is when one atom donates its valence electron to another atom, increasing the stability of both atoms. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. These ions are. Ionic Compound Definition Biology.