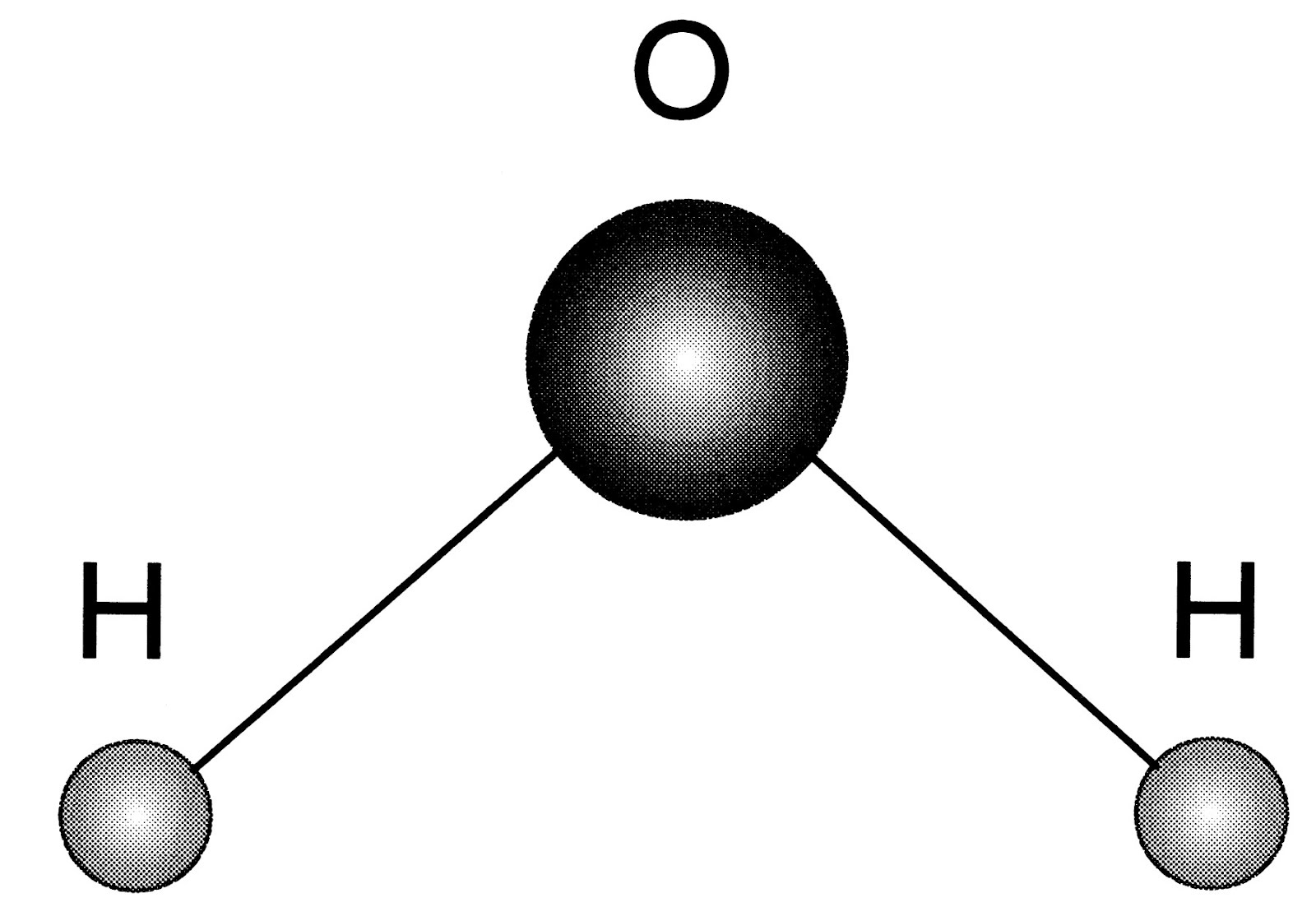
On A Water Molecule . A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. An important feature of water is its polar nature. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The oxygen atom also has two lone pairs of electrons. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature and. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. Because of the higher electronegativity of the oxygen atom, the bonds are polar.
from kgrowth.blogspot.com
The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The oxygen atom also has two lone pairs of electrons. Water is a liquid at standard ambient temperature and. An important feature of water is its polar nature. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. Because of the higher electronegativity of the oxygen atom, the bonds are polar.
KGrowth What can we know about water molecule?
On A Water Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. An important feature of water is its polar nature. The oxygen atom also has two lone pairs of electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature and.
From www.freepik.com
Premium Photo Water molecule isolated over white On A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The oxygen atom also has two lone pairs of electrons. The slightly negative particles of a compound will be attracted to water's hydrogen atoms,. On A Water Molecule.
From logyofbio.blogspot.com
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule On A Water Molecule The oxygen atom also has two lone pairs of electrons. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient. On A Water Molecule.
From www.britannica.com
water Definition, Chemical Formula, Structure, Molecule, & Facts Britannica On A Water Molecule As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. The oxygen atom also has two lone pairs of electrons. The structure has a bent molecular geometry for the two hydrogens. On A Water Molecule.
From www.e-education.psu.edu
The Configuration of the Water Molecule EARTH 111 Water Science and Society On A Water Molecule Because of the higher electronegativity of the oxygen atom, the bonds are polar. An important feature of water is its polar nature. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen. On A Water Molecule.
From www.nicerweb.com
water_molecule.html 03_02WaterMolecules_L.jpg On A Water Molecule The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The slightly. On A Water Molecule.
From lah.elearningontario.ca
SBI4U On A Water Molecule The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Water is a liquid at standard ambient temperature and. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The oxygen atom also has two lone pairs. On A Water Molecule.
From www.dreamstime.com
Abstract Water Molecules Science and Chemistry Concept Stock Illustration Illustration of On A Water Molecule The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. An important feature of water is its polar nature. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. The oxygen atom also has two lone pairs of electrons. The slightly negative particles of a compound. On A Water Molecule.
From alevelbiology.co.uk
Dipoles Of Water Molecules ALevel Biology Revision Notes On A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The oxygen atom also has two lone pairs of electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water. On A Water Molecule.
From www.alamy.com
Water molecule model Stock Photo Alamy On A Water Molecule An important feature of water is its polar nature. The oxygen atom also has two lone pairs of electrons. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. Water is a liquid at standard ambient temperature and. Water is a simple molecule consisting of one oxygen. On A Water Molecule.
From www.expii.com
Water — Molecular Structure & Bonding Expii On A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. An important feature of water is. On A Water Molecule.
From www.medicalsciencenavigator.com
Physiology of Cell Signaling On A Water Molecule Water is a liquid at standard ambient temperature and. Because of the higher electronegativity of the oxygen atom, the bonds are polar. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. The oxygen atom also has two lone pairs of electrons. As a chemical compound, a water molecule contains one. On A Water Molecule.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Water and pH On A Water Molecule The oxygen atom also has two lone pairs of electrons. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. An important feature of water is its polar nature. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. Because of the. On A Water Molecule.
From www.sciencephoto.com
Water molecule Stock Image A700/0381 Science Photo Library On A Water Molecule Water is a liquid at standard ambient temperature and. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. Because of the higher electronegativity of the oxygen atom, the bonds are polar. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few.. On A Water Molecule.
From en.citizendium.org
FileWater molecule 3D.svg Citizendium On A Water Molecule An important feature of water is its polar nature. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Water is a simple molecule consisting of one oxygen atom bonded to two. On A Water Molecule.
From www.aquaread.com
DO Meter, Probes for Measuring Dissolved Oxygen in Water On A Water Molecule An important feature of water is its polar nature. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Water is a liquid at standard ambient temperature and. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. Because of the. On A Water Molecule.
From digitalpaxton.org
water molecule On A Water Molecule The oxygen atom also has two lone pairs of electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Water is a simple molecule consisting of one oxygen atom bonded to two different. On A Water Molecule.
From www.vecteezy.com
Chemistry model molecule water H2O scientific element formula. Integrated particles natural On A Water Molecule The oxygen atom also has two lone pairs of electrons. An important feature of water is its polar nature. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. As a. On A Water Molecule.
From www.scienceabc.com
Is Water Polar Or Nonpolar? » ScienceABC On A Water Molecule A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. The oxygen atom also has two lone pairs of electrons. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. The slightly negative particles of a compound will. On A Water Molecule.
From phys.org
Water molecules dance in three On A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there. On A Water Molecule.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. On A Water Molecule The oxygen atom also has two lone pairs of electrons. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. As a chemical compound, a water molecule contains one oxygen and two. On A Water Molecule.
From cartoondealer.com
H2O Water Molecule Model And Chemical Formula Vector Illustration 126636624 On A Water Molecule Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. An important feature of water is its polar nature. The slightly negative particles of a compound will be. On A Water Molecule.
From teex.org
3d illustration with water molecule. Abstract molecule microbiology or science background On A Water Molecule The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The oxygen atom also has two lone pairs of electrons. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Water is a liquid at standard ambient temperature and. Water is a. On A Water Molecule.
From www.alamy.com
Water molecule Stock Photo Alamy On A Water Molecule A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. Water is a liquid at standard ambient temperature and. The oxygen atom also has two lone pairs of electrons. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive. On A Water Molecule.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure On A Water Molecule As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. Chemically speaking, water is a liquid substance made of. On A Water Molecule.
From pixels.com
Water Molecule Structure Photograph by Inna Bigun/science Photo Library On A Water Molecule A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. An important feature of water is its polar nature. The oxygen atom also has two lone pairs of electrons. Water is a liquid at standard ambient temperature and. The slightly negative particles of a compound will be. On A Water Molecule.
From taylorsciencegeeks.weebly.com
The Water Molecule Science News On A Water Molecule An important feature of water is its polar nature. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few. Because of the higher electronegativity of the oxygen atom, the bonds are. On A Water Molecule.
From www.thoughtco.com
Water Chemistry Definition and Properties On A Water Molecule As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The oxygen atom also has two lone pairs of electrons. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. Water is a liquid at standard ambient temperature and. The slightly. On A Water Molecule.
From kgrowth.blogspot.com
KGrowth What can we know about water molecule? On A Water Molecule As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. An important feature of water is its polar nature. A molecule is an aggregation of atomic nuclei. On A Water Molecule.
From aviewfromtheright.com
Water, Water Everywhere… Science, POLITICS, & Religion On A Water Molecule The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Because of the higher electronegativity of the oxygen atom, the bonds are polar. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The structure has a. On A Water Molecule.
From www.vecteezy.com
Chemistry model of molecule water H2O scientific elements. Integrated particles hydrogen and On A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The oxygen atom also has two lone pairs of electrons. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. An important feature of water is its polar nature. The slightly negative particles of a. On A Water Molecule.
From jeopardylabs.com
Molecular Jeopardy Template On A Water Molecule The oxygen atom also has two lone pairs of electrons. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. An important feature of water is its polar nature. Because of the higher electronegativity. On A Water Molecule.
From www.vecteezy.com
Chemistry model of molecule water H2O scientific elements. Integrated particles hydrogen and On A Water Molecule The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. The oxygen atom also has two lone pairs of electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar. An important. On A Water Molecule.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure On A Water Molecule Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. The oxygen atom also has two lone pairs of electrons. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to. On A Water Molecule.
From www.dreamstime.com
Water Molecule. Molecule Structure. Atomic H2O. Stock Vector Illustration of structure On A Water Molecule Chemically speaking, water is a liquid substance made of molecules —a single, large drop of water weighing 0.1g contains. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The slightly negative particles of a compound will be attracted. On A Water Molecule.
From depositphotos.com
The water molecule. The composition of the molecule. — Stock Vector © Olga025 96789634 On A Water Molecule The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. Because of the higher electronegativity of the oxygen atom, the bonds are polar. The slightly negative particles of a compound will be attracted to water's hydrogen atoms, while the slightly positive particles will be attracted to. Water is a simple molecule consisting of one oxygen. On A Water Molecule.