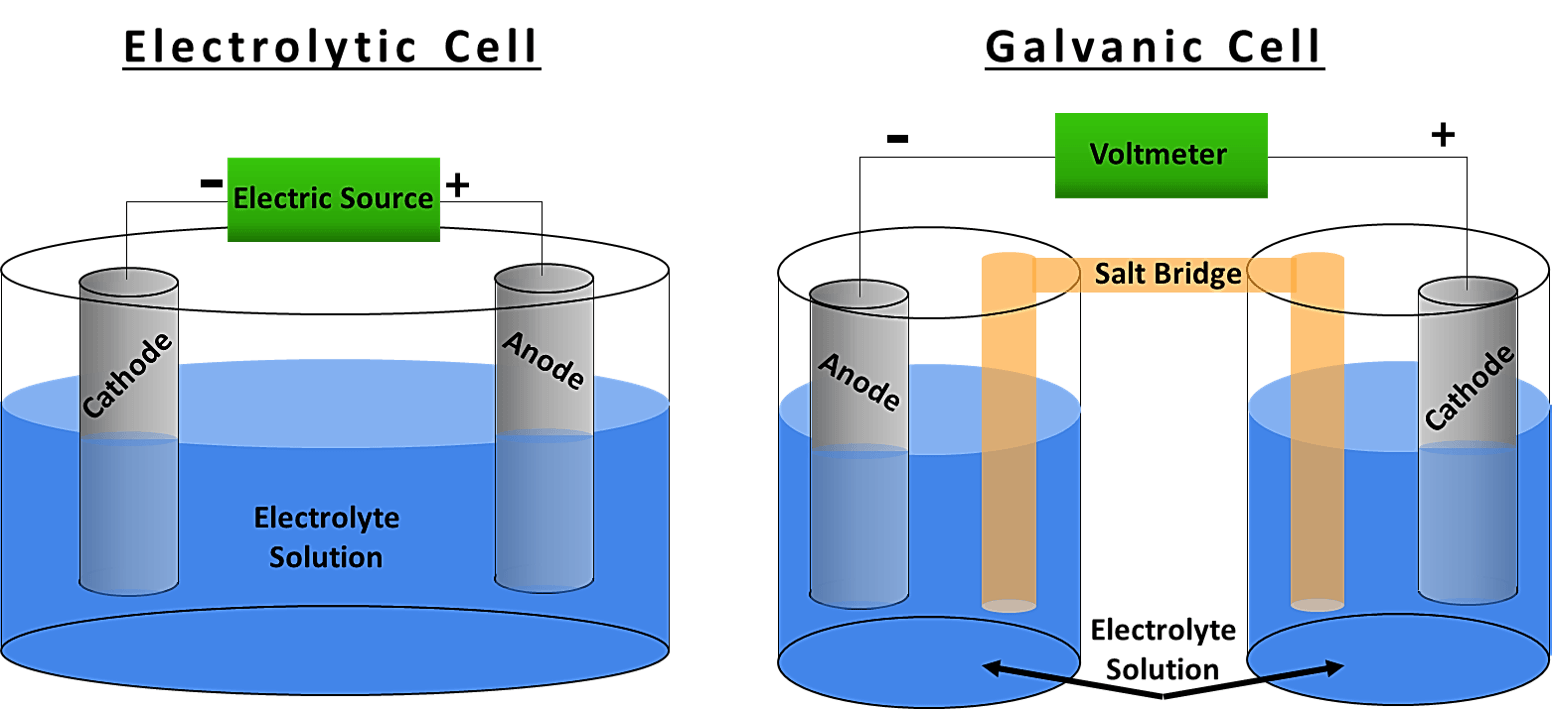
What Is The Difference Between Galvanic And Electrolytic Cell . Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The electrolytic cell uses an electric current for the. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell has a separate beaker or cell known as a half cell for each.
from www.expii.com
A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. A galvanic cell has a separate beaker or cell known as a half cell for each. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The electrolytic cell uses an electric current for the.
Electrochemical Cell — Definition & Overview Expii
What Is The Difference Between Galvanic And Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell has a separate beaker or cell known as a half cell for each. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? The electrolytic cell uses an electric current for the. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive.
From overallscience.com
Differences between electrolytic cell and voltaic cell Overall Science What Is The Difference Between Galvanic And Electrolytic Cell In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell has a separate beaker. What Is The Difference Between Galvanic And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference What Is The Difference Between Galvanic And Electrolytic Cell The electrolytic cell uses an electric current for the. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes. What Is The Difference Between Galvanic And Electrolytic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. The electrolytic cell uses an electric current for the. In. What Is The Difference Between Galvanic And Electrolytic Cell.
From testbook.com
Know Difference Between Galvanic Cells And Electrolytic Cells What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell has a separate beaker or cell known as a half cell for each. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In summary, galvanic cells generate electrical energy from a. What Is The Difference Between Galvanic And Electrolytic Cell.
From sites.google.com
Electrochemistry engineering chemistry What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? The electrolytic cell uses an electric current for the. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.pinterest.com
Difference Between Daniell Cell and Galvanic Cell Definition, How What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell has a separate beaker or cell known as a half cell for each. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.pinterest.es
What are the differences between Galvanic cell and Electrolytic cell What Is The Difference Between Galvanic And Electrolytic Cell A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.youtube.com
What is Electrochemical Cell Notation Line notation Cell Diagram What Is The Difference Between Galvanic And Electrolytic Cell Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. A galvanic cell has a. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download What Is The Difference Between Galvanic And Electrolytic Cell A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.vrogue.co
8 Difference Between Electrolytic Cell And Electroche vrogue.co What Is The Difference Between Galvanic And Electrolytic Cell The electrolytic cell uses an electric current for the. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell What Is The Difference Between Galvanic And Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require. What Is The Difference Between Galvanic And Electrolytic Cell.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells What Is The Difference Between Galvanic And Electrolytic Cell The electrolytic cell uses an electric current for the. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge,. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working What Is The Difference Between Galvanic And Electrolytic Cell A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. The electrolytic cell uses an electric current for the. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. A galvanic cell. What Is The Difference Between Galvanic And Electrolytic Cell.
From differguide.com
Difference Between Galvanic And Electrolytic Cell What Is The Difference Between Galvanic And Electrolytic Cell The electrolytic cell uses an electric current for the. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A galvanic cell is an electrochemical. What Is The Difference Between Galvanic And Electrolytic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction,. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? The electrolytic cell uses an electric current for. What Is The Difference Between Galvanic And Electrolytic Cell.
From allthedifferences.com
What Is The Difference Between Electrolytic Cells And Galvanic Cells What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell has a separate beaker or cell known as a half cell for each. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing. What Is The Difference Between Galvanic And Electrolytic Cell.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How What Is The Difference Between Galvanic And Electrolytic Cell What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? The electrolytic cell uses an electric current for the. A galvanic cell has a separate beaker or cell known as a half cell for each. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an. What Is The Difference Between Galvanic And Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell What Is The Difference Between Galvanic And Electrolytic Cell A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. The electrolytic cell uses an electric current. What Is The Difference Between Galvanic And Electrolytic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. Electrolytic cells are very similar to voltaic (galvanic). What Is The Difference Between Galvanic And Electrolytic Cell.
From www.chemca.in
Types of Electrochemical Cells Difference between galvanic cell and What Is The Difference Between Galvanic And Electrolytic Cell A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In summary, galvanic cells generate electrical energy from a. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the What Is The Difference Between Galvanic And Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The electrolytic cell uses an electric current for the. A galvanic cell. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell has a separate beaker or cell known as a half cell for each. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell is an electrochemical. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell has a separate beaker or cell known as a half cell for each. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell has a separate beaker or cell known as a half cell for each. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. A galvanic cell is an electrochemical cell in which. What Is The Difference Between Galvanic And Electrolytic Cell.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell has a separate beaker or cell known as a half cell for each. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. Galvanic cells generate electrical energy through spontaneous. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Is The Difference Between Galvanic And Electrolytic Cell In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells generate electrical energy through spontaneous. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference What Is The Difference Between Galvanic And Electrolytic Cell In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. What is the difference between a galvanic. What Is The Difference Between Galvanic And Electrolytic Cell.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell What Is The Difference Between Galvanic And Electrolytic Cell In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. What is the difference between a galvanic cell (such as a daniell cell) and an electrolytic cell? The electrolytic cell uses an electric. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.vrogue.co
Difference Between Galvanic Cells And Electrolytic Ce vrogue.co What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction,. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell What Is The Difference Between Galvanic And Electrolytic Cell A galvanic cell has a separate beaker or cell known as a half cell for each. The electrolytic cell uses an electric current for the. In summary, galvanic cells generate electrical energy from a spontaneous redox reaction, while electrolytic cells use electrical energy to drive. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require. What Is The Difference Between Galvanic And Electrolytic Cell.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint What Is The Difference Between Galvanic And Electrolytic Cell A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell has a separate beaker or cell. What Is The Difference Between Galvanic And Electrolytic Cell.