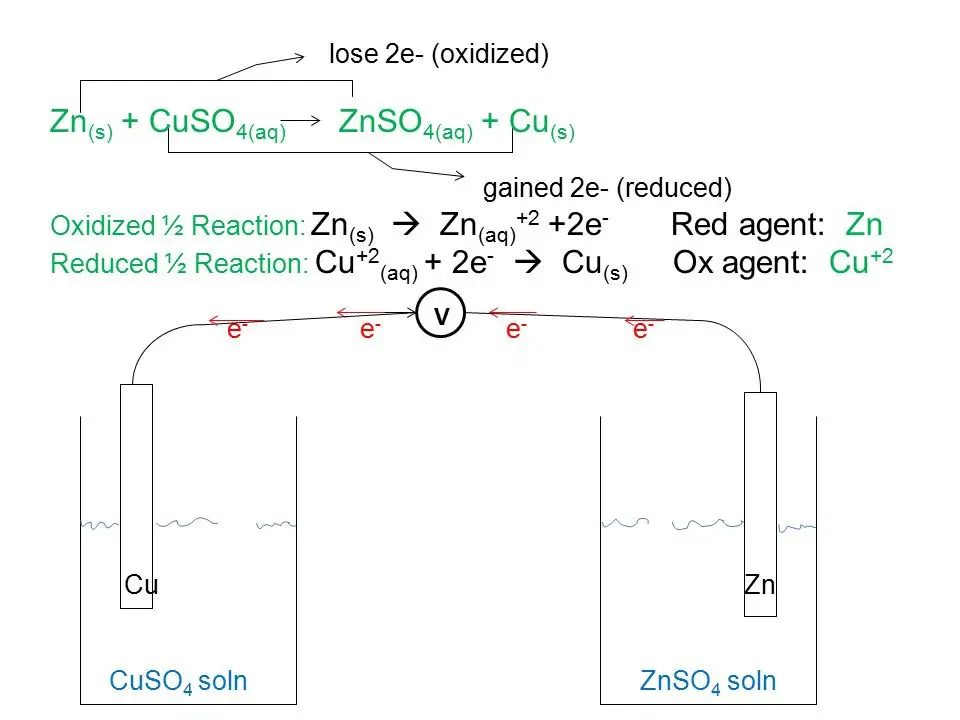
Copper And Zinc Redox Reaction . The electrons that are transferred in the reaction go directly from. in the first reaction, the copper ion is able to oxidize the zinc metal. You can find out this relative tendency. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. Reaction of zinc and copper(ii) sulfate. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. However, in the second reaction, the zinc. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. Consider the reaction of zinc metal with copper(ii) sulfate: when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken.
from www.pastpapersinside.com
when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Consider the reaction of zinc metal with copper(ii) sulfate: a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. You can find out this relative tendency. The electrons that are transferred in the reaction go directly from. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. Reaction of zinc and copper(ii) sulfate.
Redox Reaction Past Papers Inside
Copper And Zinc Redox Reaction The electrons that are transferred in the reaction go directly from. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. However, in the second reaction, the zinc. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Reaction of zinc and copper(ii) sulfate. You can find out this relative tendency. in the first reaction, the copper ion is able to oxidize the zinc metal. Consider the reaction of zinc metal with copper(ii) sulfate: when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. The electrons that are transferred in the reaction go directly from.
From classschoolhawkins.z14.web.core.windows.net
How To Recognize Redox Reactions Copper And Zinc Redox Reaction a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. You can find out this relative tendency. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. The electrons that are transferred in. Copper And Zinc Redox Reaction.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Khan Academy YouTube Copper And Zinc Redox Reaction However, in the second reaction, the zinc. Reaction of zinc and copper(ii) sulfate. Consider the reaction of zinc metal with copper(ii) sulfate: If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. the test tube reaction happens because of the relative tendency of the zinc and copper. Copper And Zinc Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Copper And Zinc Redox Reaction the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. in the first reaction, the copper ion is able to oxidize the zinc metal. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. when a strip of zinc metal. Copper And Zinc Redox Reaction.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Copper And Zinc Redox Reaction the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate. Copper And Zinc Redox Reaction.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate YouTube Copper And Zinc Redox Reaction If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Reaction of zinc and copper(ii) sulfate. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. The electrons that are transferred in the reaction go directly from. when a strip. Copper And Zinc Redox Reaction.
From www.nagwa.com
Question Video Describing How Oxidation Changes a Chemical Species in the Reaction between Copper And Zinc Redox Reaction Consider the reaction of zinc metal with copper(ii) sulfate: Reaction of zinc and copper(ii) sulfate. You can find out this relative tendency. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and.. Copper And Zinc Redox Reaction.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Redox (II) Oxidation and Reduction Copper And Zinc Redox Reaction a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate. Copper And Zinc Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Copper And Zinc Redox Reaction You can find out this relative tendency. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. If left in the solution for. Copper And Zinc Redox Reaction.
From www.reddit.com
The result of an Oxidationreduction reaction between copper and Zinc Chloride r/chemistry Copper And Zinc Redox Reaction the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. a simple redox reaction occurs when copper metal is immersed in a. Copper And Zinc Redox Reaction.
From www.youtube.com
Redox reactions of transition metals YouTube Copper And Zinc Redox Reaction The electrons that are transferred in the reaction go directly from. Consider the reaction of zinc metal with copper(ii) sulfate: the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. You can find out this relative. Copper And Zinc Redox Reaction.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper And Zinc Redox Reaction the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. You can find out this relative tendency. Consider the reaction of zinc metal with copper(ii) sulfate: the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. in the first reaction,. Copper And Zinc Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Copper And Zinc Redox Reaction The electrons that are transferred in the reaction go directly from. a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. in the first reaction, the copper ion is able to oxidize the zinc metal.. Copper And Zinc Redox Reaction.
From www.learner.org
A Redox Reaction Using Copper and Zinc (animation) Annenberg Learner Copper And Zinc Redox Reaction the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Consider the reaction of zinc metal with copper(ii). Copper And Zinc Redox Reaction.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation ID4349314 Copper And Zinc Redox Reaction a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. Consider the reaction of zinc metal with copper(ii) sulfate: when a strip of zinc metal is placed into a blue solution. Copper And Zinc Redox Reaction.
From pendulumedu.com
Oxidation and Reduction Redox Reactions, Definitions, Examples Copper And Zinc Redox Reaction If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. You can find out this relative tendency. the test tube reaction happens because of the relative tendency of the zinc. Copper And Zinc Redox Reaction.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Copper And Zinc Redox Reaction the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Reaction of zinc and copper(ii) sulfate. The electrons that are transferred in the reaction go directly from. You can find out. Copper And Zinc Redox Reaction.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part... Course Hero Copper And Zinc Redox Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. Consider the reaction of zinc metal. Copper And Zinc Redox Reaction.
From www.learner.org
A Redox Reaction Using Copper and Zinc (animation) Annenberg Learner Copper And Zinc Redox Reaction Reaction of zinc and copper(ii) sulfate. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. the. Copper And Zinc Redox Reaction.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation, free download ID4349314 Copper And Zinc Redox Reaction Reaction of zinc and copper(ii) sulfate. in the first reaction, the copper ion is able to oxidize the zinc metal. You can find out this relative tendency. The electrons that are transferred in the reaction go directly from. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. Consider the reaction of. Copper And Zinc Redox Reaction.
From www.slideshare.net
Redox= quiz part 1 with answers Copper And Zinc Redox Reaction The electrons that are transferred in the reaction go directly from. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. Consider the reaction of zinc metal with copper(ii) sulfate: However,. Copper And Zinc Redox Reaction.
From slideplayer.com
RedOx Reactions & Electrochemistry ppt download Copper And Zinc Redox Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. You can find out this relative tendency. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form. Copper And Zinc Redox Reaction.
From saylordotorg.github.io
Applications of Redox Reactions Voltaic Cells Copper And Zinc Redox Reaction the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. in the first reaction, the copper ion is able to oxidize the zinc metal. You. Copper And Zinc Redox Reaction.
From www.youtube.com
Copper oxide + zinc powder redox reactions YouTube Copper And Zinc Redox Reaction Reaction of zinc and copper(ii) sulfate. The electrons that are transferred in the reaction go directly from. You can find out this relative tendency. in the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc. when a strip of zinc metal is placed into a blue solution of. Copper And Zinc Redox Reaction.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Aakash AESL Copper And Zinc Redox Reaction the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. in the first reaction, the copper ion is able to oxidize the zinc metal. You can find out this relative tendency. If left in the solution for a longer period of time, the zinc will gradually decay. Copper And Zinc Redox Reaction.
From byjus.com
14.During indirect redox reaction why there is a release of zinc ions from zinc rod and not of Copper And Zinc Redox Reaction when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. The electrons that are transferred in the reaction. Copper And Zinc Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction copper zinc Fundamental Photographs The Art of Science Copper And Zinc Redox Reaction the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. You can find out this relative tendency. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. when a strip of zinc metal is placed into a blue solution of. Copper And Zinc Redox Reaction.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation, free download ID4349314 Copper And Zinc Redox Reaction Reaction of zinc and copper(ii) sulfate. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. in the first reaction, the copper ion is able to oxidize the zinc metal. . Copper And Zinc Redox Reaction.
From sciencenotes.org
Redox Reactions Identify and Balance Oxidation and Reduction Copper And Zinc Redox Reaction when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. Consider the reaction of zinc metal with copper(ii) sulfate:. Copper And Zinc Redox Reaction.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Copper And Zinc Redox Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. You can find out this relative tendency. the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. Reaction of zinc and copper(ii) sulfate. a simple redox reaction occurs when copper metal is immersed in a solution of. Copper And Zinc Redox Reaction.
From www.numerade.com
SOLVED Copper reaction Draw the copper half reaction going from copper solid to copper II ion Copper And Zinc Redox Reaction Consider the reaction of zinc metal with copper(ii) sulfate: The electrons that are transferred in the reaction go directly from. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. However, in the second reaction, the zinc. in the first reaction, the copper ion is able to. Copper And Zinc Redox Reaction.
From www.youtube.com
Redox demo of the redox reaction of copper and zinc YouTube Copper And Zinc Redox Reaction If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. in the first reaction, the copper ion is able to oxidize the zinc metal. the. Copper And Zinc Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Copper And Zinc Redox Reaction a simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Reaction of zinc and copper(ii) sulfate. in the first reaction, the copper ion is able to oxidize the zinc metal. The electrons that are transferred in the reaction go directly from. Consider the reaction of zinc metal with copper(ii) sulfate: However, in. Copper And Zinc Redox Reaction.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation, free download ID1733600 Copper And Zinc Redox Reaction a redox reaction using copper and zinc (animation) in this demonstration, dissolved copper ions come in contact with zinc, and. Reaction of zinc and copper(ii) sulfate. However, in the second reaction, the zinc. the test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. If left in the. Copper And Zinc Redox Reaction.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free download ID1195783 Copper And Zinc Redox Reaction the reaction of zinc metal with copper(ii) ions is called a direct redox process or reaction. in the first reaction, the copper ion is able to oxidize the zinc metal. Consider the reaction of zinc metal with copper(ii) sulfate: The electrons that are transferred in the reaction go directly from. a simple redox reaction occurs when copper. Copper And Zinc Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs The Art of Science Copper And Zinc Redox Reaction If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. The electrons that are transferred in the reaction. Copper And Zinc Redox Reaction.