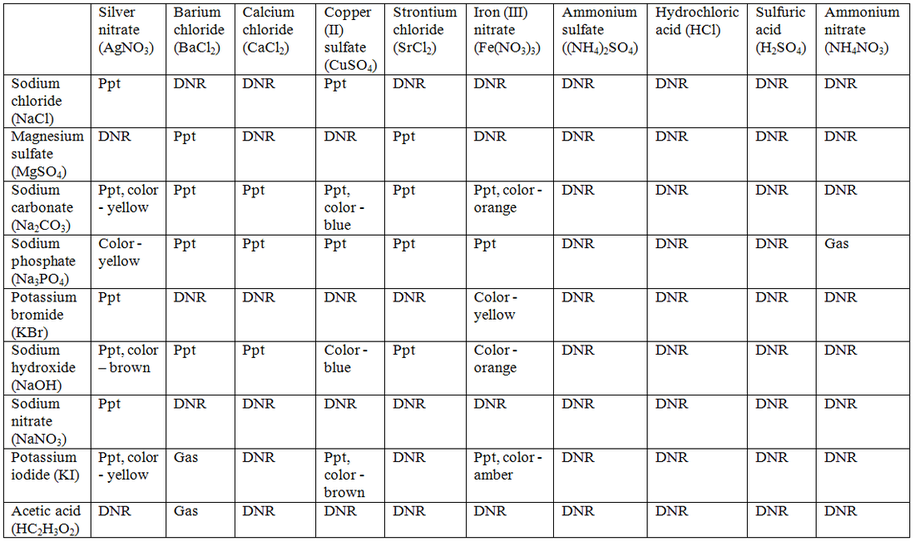
Table Salt Net Ionic Equation . Enter an equation of an ionic chemical equation and press the balance button. Reactions of salts with water. Let’s use the reaction between sodium chloride and silver nitrate as an example. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. Next, we write the chemical equation as a complete ionic equation. The first step to writing a net ionic equation is balancing the chemical equation present. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. For the net ionic equation, start with. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write.
from katiem728.weebly.com
Reactions of salts with water. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Next, we write the chemical equation as a complete ionic equation. Enter an equation of an ionic chemical equation and press the balance button. For the net ionic equation, start with. A net ionic equation is the most accurate representation of the actual chemical process that occurs. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. Let’s use the reaction between sodium chloride and silver nitrate as an example. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write.
Net Ionic Equation Lab Katie's AP Chemistry site
Table Salt Net Ionic Equation 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Let’s use the reaction between sodium chloride and silver nitrate as an example. Reactions of salts with water. For the net ionic equation, start with. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. A net ionic equation is the most accurate representation of the actual chemical process that occurs. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. Enter an equation of an ionic chemical equation and press the balance button. Next, we write the chemical equation as a complete ionic equation. The first step to writing a net ionic equation is balancing the chemical equation present.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Table Salt Net Ionic Equation Reactions of salts with water. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. Next, we write the chemical equation as a complete ionic equation. The first step. Table Salt Net Ionic Equation.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Table Salt Net Ionic Equation For the net ionic equation, start with. Next, we write the chemical equation as a complete ionic equation. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. A net ionic equation is the most accurate representation. Table Salt Net Ionic Equation.
From www.chegg.com
In Table 1, write the net ionic equation for the Table Salt Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. The first step to writing a net ionic equation is balancing the chemical equation present. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. Let’s use the reaction between sodium chloride and silver nitrate as an example.. Table Salt Net Ionic Equation.
From www.numerade.com
SOLVED Problem Write balanced molecular; total ionic. and net ionic Table Salt Net Ionic Equation Let’s use the reaction between sodium chloride and silver nitrate as an example. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The first step to writing a net ionic equation is balancing the chemical equation present. The net ionic equation tell you at a glance which ions influence product formation and whether. Table Salt Net Ionic Equation.
From chemistryfromscratch.org
V2.6 Table Salt Net Ionic Equation Let’s use the reaction between sodium chloride and silver nitrate as an example. For the net ionic equation, start with. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Reactions of salts with water. Next, we write the chemical equation as. Table Salt Net Ionic Equation.
From apchemsite.wordpress.com
Net Ionic Equation Chemistry Geeks Table Salt Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. The first step to writing a net ionic equation is balancing the chemical equation present. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular. Table Salt Net Ionic Equation.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Table Salt Net Ionic Equation Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. For the net ionic equation, start with. Reactions of salts with water. The first step to writing a net ionic equation is balancing the chemical equation present. Next, we write the chemical equation as a complete ionic equation. The net ionic equation tell you at a. Table Salt Net Ionic Equation.
From www.bartleby.com
Answered Write a balanced net ionic equation to… bartleby Table Salt Net Ionic Equation Reactions of salts with water. Let’s use the reaction between sodium chloride and silver nitrate as an example. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The net ionic. Table Salt Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Pb(NO3)2 + KI = KNO3 + PbI2 Table Salt Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Next, we write the chemical equation as a complete ionic equation. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The. Table Salt Net Ionic Equation.
From www.chegg.com
Solved Write a balanced net ionic equation to show why the Table Salt Net Ionic Equation A net ionic equation is the most accurate representation of the actual chemical process that occurs. Next, we write the chemical equation as a complete ionic equation. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. The net ionic equation tell you at a glance which ions influence product formation and whether or not there. Table Salt Net Ionic Equation.
From www.tessshebaylo.com
Ammonium Iodide Dissolved In Water Net Ionic Equation Tessshebaylo Table Salt Net Ionic Equation Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Let’s use the reaction between sodium chloride and silver nitrate as an example. The net ionic equation tell you at a glance which ions influence product formation and whether or not there. Table Salt Net Ionic Equation.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Table Salt Net Ionic Equation For the net ionic equation, start with. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Next, we write the. Table Salt Net Ionic Equation.
From www.youtube.com
Is Table Salt (NaCl ) Ionic or Covalent/Molecular? YouTube Table Salt Net Ionic Equation Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. The first step to writing a net ionic equation is balancing the chemical equation present. Reactions of salts with water. Let’s use the reaction between sodium chloride and silver nitrate as an example. Enter an equation of an ionic chemical equation and press the balance button.. Table Salt Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for AgNO3 + NaCl = AgCl + NaNO3 Table Salt Net Ionic Equation For the net ionic equation, start with. Reactions of salts with water. The first step to writing a net ionic equation is balancing the chemical equation present. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Next, we write the chemical equation as a complete ionic. Table Salt Net Ionic Equation.
From stock.adobe.com
Sodium chloride (table salt), chemical structure. Skeletal formula Table Salt Net Ionic Equation Next, we write the chemical equation as a complete ionic equation. Reactions of salts with water. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The first step to writing a net ionic equation is balancing the chemical equation present. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can. Table Salt Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for CaCl2 + Na2CO3 = CaCO3 + NaCl Table Salt Net Ionic Equation The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. For the net ionic equation, start with. Let’s use the reaction between sodium chloride and silver nitrate as an example. The first step. Table Salt Net Ionic Equation.
From elchoroukhost.net
Write The Chemical Formulas Of Two Ions When Table Salt Dissolved In Table Salt Net Ionic Equation Reactions of salts with water. For the net ionic equation, start with. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Enter an equation of an ionic chemical equation and press the balance button. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Hydrolysis as. Table Salt Net Ionic Equation.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Table Salt Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Let’s use the reaction between sodium chloride and silver nitrate as an example. Hydrolysis as applied to water. Table Salt Net Ionic Equation.
From www.numerade.com
SOLVED Question 3 (4 marks; 2 marks each) Sulphuric acid is completely Table Salt Net Ionic Equation The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. The first step to writing a net ionic equation is balancing the chemical equation present. For the net ionic equation, start with. Next, we write the chemical equation as a complete ionic equation. The net ionic equation. Table Salt Net Ionic Equation.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Table Salt Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Reactions of salts with water. 1) molecular, 2) ionic, 3) net ionic • here. Table Salt Net Ionic Equation.
From www.reddit.com
Write the net ionic equation in which the slightly soluble salt Table Salt Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly.. Table Salt Net Ionic Equation.
From twobirdsfourhands.com
Chemical Formula For Iodized Table Salt Two Birds Home Table Salt Net Ionic Equation For the net ionic equation, start with. Enter an equation of an ionic chemical equation and press the balance button. Next, we write the chemical equation as a complete ionic equation. The first step to writing a net ionic equation is balancing the chemical equation present. Let’s use the reaction between sodium chloride and silver nitrate as an example. The. Table Salt Net Ionic Equation.
From socratic.org
Which of the following substances are insoluble in water? Socratic Table Salt Net Ionic Equation Next, we write the chemical equation as a complete ionic equation. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. Reactions of salts with water. 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. For the net. Table Salt Net Ionic Equation.
From katiem728.weebly.com
Net Ionic Equation Lab Katie's AP Chemistry site Table Salt Net Ionic Equation Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. Let’s use the reaction between sodium chloride and silver nitrate as an example. Next, we write the chemical equation as a complete ionic equation. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. The net ionic equation tell you at a glance. Table Salt Net Ionic Equation.
From quizizz.com
Year 1011 IGSCE Solubility and Precipitation Quizizz Table Salt Net Ionic Equation Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. Next, we write the chemical equation as a complete ionic equation. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Reactions of salts with water. Enter an equation of an ionic chemical. Table Salt Net Ionic Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Table Salt Net Ionic Equation 1) molecular, 2) ionic, 3) net ionic • here is a typical molecular equation: Reactions of salts with water. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Next, we write the chemical equation as a complete ionic equation. Hydrolysis as applied to water solutions of inorganic compounds, can be. Table Salt Net Ionic Equation.
From elchoroukhost.net
What Is The Chemical Equation For Table Salt Elcho Table Table Salt Net Ionic Equation The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. The first step to writing a net ionic equation is balancing the chemical equation present. Next, we write the chemical equation as a complete. Table Salt Net Ionic Equation.
From treatybottle13.pythonanywhere.com
Favorite Net Ionic Equations Calculator Regents Physics Reference Table Table Salt Net Ionic Equation Reactions of salts with water. Next, we write the chemical equation as a complete ionic equation. The first step to writing a net ionic equation is balancing the chemical equation present. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Let’s use the reaction between sodium. Table Salt Net Ionic Equation.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Table Salt Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Enter an equation of an ionic chemical equation and press the balance button. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. Let’s use the reaction between. Table Salt Net Ionic Equation.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Table Salt Net Ionic Equation Next, we write the chemical equation as a complete ionic equation. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The net ionic equation tell you at a glance which ions influence product formation and whether or not there is a solid present. For the net ionic equation, start with. Let’s use the. Table Salt Net Ionic Equation.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID6901289 Table Salt Net Ionic Equation Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. Reactions of salts with water. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The first step to writing a net ionic equation is balancing the chemical equation present. The net ionic equation is the chemical equation that shows. Table Salt Net Ionic Equation.
From cabinet.matttroy.net
Is Table Salt A Molecule Or Compound Matttroy Table Salt Net Ionic Equation Next, we write the chemical equation as a complete ionic equation. Reactions of salts with water. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write. 1) molecular, 2) ionic, 3) net ionic • here is a. Table Salt Net Ionic Equation.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Table Salt Net Ionic Equation Hydrolysis as applied to water solutions of inorganic compounds, can be defined as. The first step to writing a net ionic equation is balancing the chemical equation present. Enter an equation of an ionic chemical equation and press the balance button. Reactions of salts with water. Cd(no 3) 2(aq) + na 2s(aq) →cds(s) + 2nano 3(aq) • we can write.. Table Salt Net Ionic Equation.
From apchemistrylabkrebs.weebly.com
Net Ionic Equations Lab AP Chemistry Shelly Oh Table Salt Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly. For the net ionic equation, start with. A net ionic equation is the most accurate representation of the actual chemical process that occurs. Next, we write the chemical equation as a complete ionic equation. Enter an equation of an ionic chemical. Table Salt Net Ionic Equation.
From socratic.org
What is the molecular, total ionic, and net ionic equations of these Table Salt Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. A net ionic equation is the most accurate representation of the actual chemical process that occurs. Next, we write the chemical equation as a complete ionic equation. Let’s use the reaction between sodium chloride and silver nitrate as an example. The net ionic equation tell you at. Table Salt Net Ionic Equation.