Atomic Emissions Spectra Lab . By the end of this lab, students should be able to: To practice writing electron configurations for these (and other) elements. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Identify that all elements have unique line emission spectra. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. You will observe spectra from several different light sources and compare them. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. To know the relationship between atomic emission spectra and the electronic structure of atoms.
from ciderhousetech.com.au
Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Identify that all elements have unique line emission spectra. You will observe spectra from several different light sources and compare them. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. To practice writing electron configurations for these (and other) elements. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. By the end of this lab, students should be able to: To know the relationship between atomic emission spectra and the electronic structure of atoms. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,.
Atomic Spectra Experiment Cider House Tech
Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Identify that all elements have unique line emission spectra. To know the relationship between atomic emission spectra and the electronic structure of atoms. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. To practice writing electron configurations for these (and other) elements. You will observe spectra from several different light sources and compare them. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. By the end of this lab, students should be able to:
From learninglistlang.z19.web.core.windows.net
Atomic Spectra Worksheet Answers Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. To know the relationship between atomic emission spectra and the electronic structure of atoms. Identify that all elements have unique line emission spectra. By the end of this lab, students should be able to: To practice writing electron configurations for these (and other) elements. In this lab you. Atomic Emissions Spectra Lab.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Atomic Emissions Spectra Lab To practice writing electron configurations for these (and other) elements. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. You will observe spectra from several different light sources and compare them. By the end of this lab, students should be able to: Identify. Atomic Emissions Spectra Lab.
From chemcollective.org
CHEM1315 Lab 8 Atomic Spectrum Atomic Emissions Spectra Lab Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. Identify that all elements have unique line emission spectra. An atomic emission spectrum is the pattern of lines formed when light. Atomic Emissions Spectra Lab.
From rightmetal.weebly.com
Atomic emission spectrum chemistry definition rightmetal Atomic Emissions Spectra Lab Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. To know the relationship between atomic emission spectra and the electronic structure of atoms. To practice writing electron configurations for these (and other) elements. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies. Atomic Emissions Spectra Lab.
From triphandarmo.weebly.com
Atomicspectraanswerkey Atomic Emissions Spectra Lab Identify that all elements have unique line emission spectra. You will observe spectra from several different light sources and compare them. To practice writing electron configurations for these (and other) elements. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. By the end of this lab, students should be able to: In this lab you will. Atomic Emissions Spectra Lab.
From www.studocu.com
Atomic Emission Spectra Lab Report Lab Report Atomic Emission Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. By the end of this lab, students should be able to: In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. To. Atomic Emissions Spectra Lab.
From studylib.net
Lab Report Atomic Emission Spectra Atomic Emissions Spectra Lab An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. To know the relationship between atomic emission spectra and the electronic structure of atoms. To practice writing electron configurations for these (and other) elements. Identify that all elements have unique line emission spectra.. Atomic Emissions Spectra Lab.
From observatory.uncc.edu
UNC Charlotte Observatory Atomic spectra lab spectra ID Atomic Emissions Spectra Lab By the end of this lab, students should be able to: Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained. Atomic Emissions Spectra Lab.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Atomic Emissions Spectra Lab Identify that all elements have unique line emission spectra. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. To know the relationship between atomic emission spectra and the electronic structure. Atomic Emissions Spectra Lab.
From www.slideserve.com
PPT Atomic Emission Spectra PowerPoint Presentation, free download Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. To practice writing electron configurations for these (and other) elements. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an. Atomic Emissions Spectra Lab.
From thekidsworksheet.com
Atomic Emission Spectra And The Quantum Mechanical Model Worksheet Atomic Emissions Spectra Lab In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. You will observe spectra from several different light sources and compare them. To practice writing electron configurations for these (and other) elements. To know the relationship between atomic emission spectra and the electronic structure of atoms. An. Atomic Emissions Spectra Lab.
From mavink.com
Visible Line Spectrum Of Hydrogen Atomic Emissions Spectra Lab Identify that all elements have unique line emission spectra. To practice writing electron configurations for these (and other) elements. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by. Atomic Emissions Spectra Lab.
From www.vernier.com
Spectrum of Atomic Hydrogen > Experiment 21 from Advanced Physics with Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. To practice writing electron configurations for these (and other) elements. By the end of this lab, students should be able to: In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. Build and calibrate a. Atomic Emissions Spectra Lab.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Atomic Emissions Spectra Lab An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. You will observe spectra from several different light sources and compare them. To practice writing electron configurations for these (and. Atomic Emissions Spectra Lab.
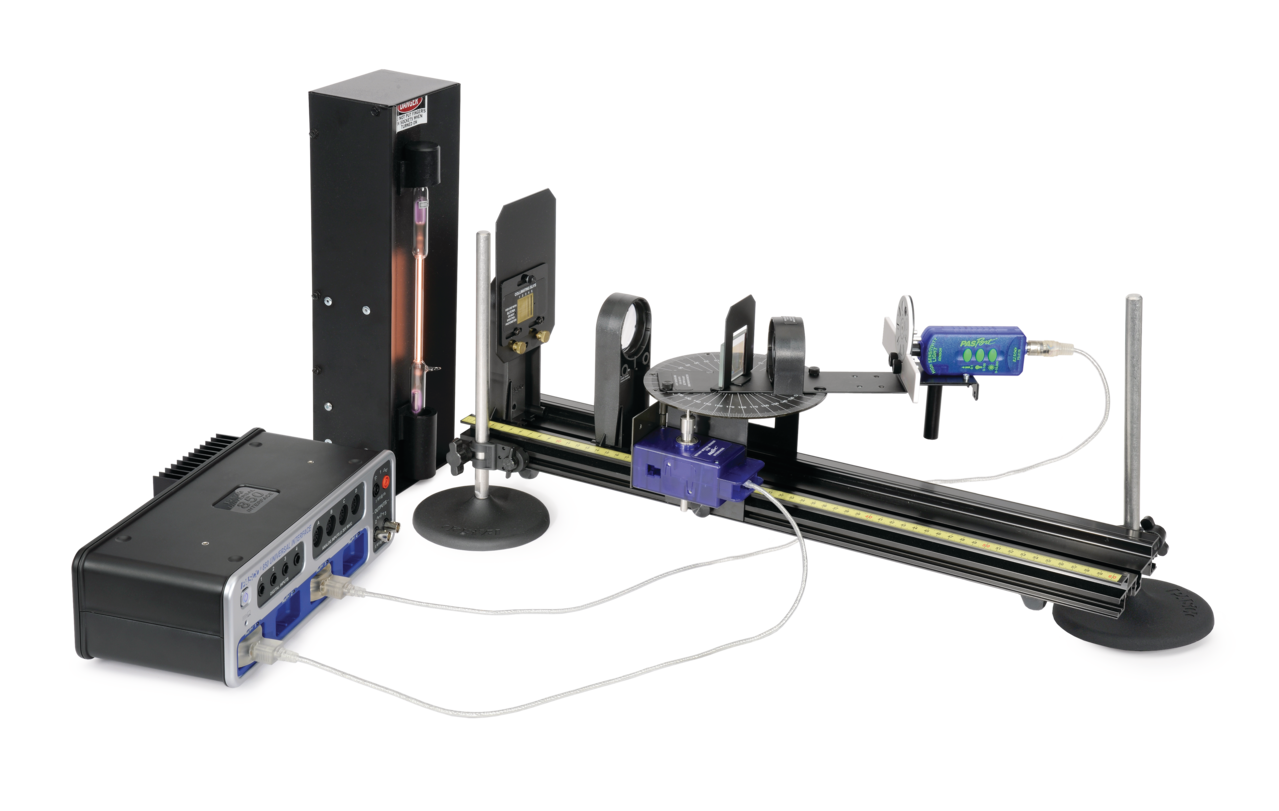
From www.pasco.com
Atomic Spectra Experiment EX5546 Products PASCO Atomic Emissions Spectra Lab To know the relationship between atomic emission spectra and the electronic structure of atoms. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In this lab you will use. Atomic Emissions Spectra Lab.
From ecampusontario.pressbooks.pub
8.2 Atomic Spectra General Chemistry for GeeGees Atomic Emissions Spectra Lab The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg. Atomic Emissions Spectra Lab.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. To practice writing electron configurations for these (and other) elements. Identify that all elements have unique line emission spectra. To know the. Atomic Emissions Spectra Lab.
From chem.libretexts.org
14B Atomic Emissions Spectra Pizza Box Version (Experiment Atomic Emissions Spectra Lab An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. By the end of this lab, students should be able to: In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula. Atomic Emissions Spectra Lab.
From heliumoseiha.blogspot.com
Helium Helium Emission Spectrum Atomic Emissions Spectra Lab An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. By the end of this lab, students should be able. Atomic Emissions Spectra Lab.
From chem.libretexts.org
6.3 Line Spectra and the Bohr Model Chemistry LibreTexts Atomic Emissions Spectra Lab The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Build and calibrate a simple spectroscope capable. Atomic Emissions Spectra Lab.
From quizzfullmarks77.z19.web.core.windows.net
Atomic Emission Spectra Lab Pdf Atomic Emissions Spectra Lab The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. By the end of this lab, students should be able to: Identify that all elements have unique line emission spectra. To. Atomic Emissions Spectra Lab.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. By the end of this lab, students should be able to: An atomic emission spectrum is the pattern of lines formed when. Atomic Emissions Spectra Lab.
From mavink.com
Visible Line Spectrum Of Hydrogen Atomic Emissions Spectra Lab To know the relationship between atomic emission spectra and the electronic structure of atoms. By the end of this lab, students should be able to: To practice writing electron configurations for these (and other) elements. Identify that all elements have unique line emission spectra. An atomic emission spectrum is the pattern of lines formed when light passes through a prism. Atomic Emissions Spectra Lab.
From chem.libretexts.org
1.4 The Hydrogen Atomic Spectrum Chemistry LibreTexts Atomic Emissions Spectra Lab Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. By the end of this lab, students should be able to: An atomic emission spectrum is the pattern of lines formed. Atomic Emissions Spectra Lab.
From jeffery-has-robertson.blogspot.com
Describe the Cause of Atomic Emission Spectrum of an Element Jeffery Atomic Emissions Spectra Lab Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. You will observe spectra from several different light sources and compare them. In this lab you will use a diffraction. Atomic Emissions Spectra Lab.
From triphandarmo.weebly.com
Atomicspectraanswerkey Atomic Emissions Spectra Lab An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. To know the relationship between atomic emission spectra and the electronic structure of atoms. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon. Atomic Emissions Spectra Lab.
From www.chegg.com
Solved EXPERIMENT 8 ATOMIC EMISSION SPECTRA Name Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. To know the relationship between atomic emission spectra and the electronic structure of atoms. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Identify that all elements have unique line emission spectra. The discrete bright (dark) lines in the emission (absorption) spectrum can be. Atomic Emissions Spectra Lab.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Atomic Emissions Spectra Lab You will observe spectra from several different light sources and compare them. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. By the end of this lab, students should be able to: To practice writing electron configurations for these (and other) elements. Identify that all elements have unique line emission spectra. To know the relationship between. Atomic Emissions Spectra Lab.
From www.youtube.com
2.2 The hydrogen emission spectrum YouTube Atomic Emissions Spectra Lab The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. To know the relationship between atomic emission. Atomic Emissions Spectra Lab.
From www.youtube.com
Atomic Emission Spectra Lab YouTube Atomic Emissions Spectra Lab By the end of this lab, students should be able to: To know the relationship between atomic emission spectra and the electronic structure of atoms. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. The discrete bright (dark) lines in the emission (absorption) spectrum can be. Atomic Emissions Spectra Lab.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Atomic Emissions Spectra Lab The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. To practice writing electron configurations for these (and other) elements. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Identify that all elements have unique line emission spectra. An atomic. Atomic Emissions Spectra Lab.
From ciderhousetech.com.au
Atomic Spectra Experiment Cider House Tech Atomic Emissions Spectra Lab Identify that all elements have unique line emission spectra. The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. To know the relationship between atomic emission spectra and the electronic structure of atoms. In this lab you will use a diffraction based spectrometer to. Atomic Emissions Spectra Lab.
From www.studocu.com
Atomic Emission Spectra Lab Report To create a basis for comparison Atomic Emissions Spectra Lab An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the rydberg formula to. Identify that all elements have unique line emission spectra. You. Atomic Emissions Spectra Lab.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce Atomic Emissions Spectra Lab An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the. Atomic Emissions Spectra Lab.
From www.pinterest.es
emission spectra periodic table Google Search AP Chem 5 Atomic Atomic Emissions Spectra Lab The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an atom,. To know the relationship between atomic emission spectra and the electronic structure of atoms. By the end of this lab, students should be able to: An atomic emission spectrum is the pattern of lines. Atomic Emissions Spectra Lab.