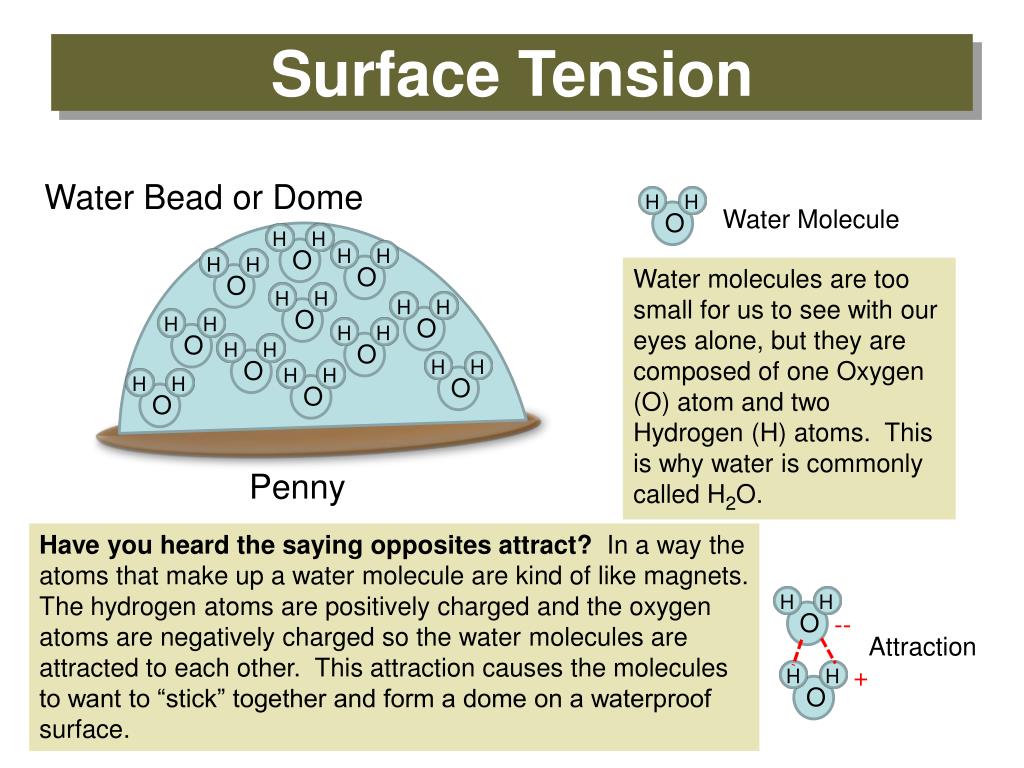
What Is Responsible For The High Surface Tension Of Water . The surface tension of water is 72 dynes/cm at 25°c. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. Since these intermolecular forces vary. The cohesive forces between liquid molecules are responsible for the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation of the presence of. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The surface tension of water decreases significantly with.
from www.slideserve.com
Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Next to mercury, water has the highest surface tension of all commonly occurring liquids. The surface tension of water decreases significantly with. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is a manifestation of the presence of. The surface tension of water is 72 dynes/cm at 25°c. Since these intermolecular forces vary. The cohesive forces between liquid molecules are responsible for the. It would take a force of 72 dynes to break a surface film of water 1 cm long.
PPT Surface Tension PowerPoint Presentation, free download ID3106425
What Is Responsible For The High Surface Tension Of Water The surface tension of water is 72 dynes/cm at 25°c. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Since these intermolecular forces vary. Surface tension is a manifestation of the presence of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules are responsible for the. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Is Responsible For The High Surface Tension Of Water The cohesive forces between liquid molecules are responsible for the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The surface tension of water is 72 dynes/cm at 25°c. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is a manifestation of. What Is Responsible For The High Surface Tension Of Water.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download What Is Responsible For The High Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. It would take a force of 72 dynes. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Seawater Chemistry PowerPoint Presentation, free download ID What Is Responsible For The High Surface Tension Of Water The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since these intermolecular forces vary. Surface tension is a manifestation of the presence of. The surface. What Is Responsible For The High Surface Tension Of Water.
From factsandhistory.com
Why Does Water Feel Like Concrete When You Belly Flop Into It What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Since these intermolecular forces vary. Surface tension is a manifestation of the presence of. The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area. What Is Responsible For The High Surface Tension Of Water.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) What Is Responsible For The High Surface Tension Of Water Since these intermolecular forces vary. The cohesive forces between liquid molecules are responsible for the. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. The surface tension of water decreases significantly with. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is a. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint What Is Responsible For The High Surface Tension Of Water The cohesive forces between liquid molecules are responsible for the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension because hydrogen bonds among water molecules resist stretching. What Is Responsible For The High Surface Tension Of Water.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download What Is Responsible For The High Surface Tension Of Water Surface tension is a manifestation of the presence of. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1. What Is Responsible For The High Surface Tension Of Water.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co What Is Responsible For The High Surface Tension Of Water Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a manifestation of the presence of. The cohesive forces between liquid molecules are responsible for the. The surface tension. What Is Responsible For The High Surface Tension Of Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Is Responsible For The High Surface Tension Of Water The cohesive forces between liquid molecules are responsible for the. Surface tension is a manifestation of the presence of. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. The surface tension of water is 72 dynes/cm at 25°c. Since these intermolecular forces vary. The surface tension of water decreases significantly with. Water has a. What Is Responsible For The High Surface Tension Of Water.
From studyfullirene.z21.web.core.windows.net
Surface Tension Of Water Worksheet What Is Responsible For The High Surface Tension Of Water The cohesive forces between liquid molecules are responsible for the. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is a manifestation of the presence of. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy, or work,. What Is Responsible For The High Surface Tension Of Water.
From www.youtube.com
Surface Tension of Water Explained YouTube What Is Responsible For The High Surface Tension Of Water It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. The cohesive forces between liquid molecules are responsible for the. Next to mercury, water has the highest. What Is Responsible For The High Surface Tension Of Water.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. Since these intermolecular forces vary. The surface tension of water is 72 dynes/cm at 25°c. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation of. What Is Responsible For The High Surface Tension Of Water.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Is Responsible For The High Surface Tension Of Water Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since these intermolecular forces vary. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is a manifestation of the presence of. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the. What Is Responsible For The High Surface Tension Of Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What Is Responsible For The High Surface Tension Of Water Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation of the presence of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water decreases significantly with. Water has a surface tension of 0.07275 joule per. What Is Responsible For The High Surface Tension Of Water.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The cohesive forces between liquid molecules are responsible for the. The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 What Is Responsible For The High Surface Tension Of Water The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. The cohesive forces between liquid molecules are responsible for the. Next to. What Is Responsible For The High Surface Tension Of Water.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse What Is Responsible For The High Surface Tension Of Water The surface tension of water is 72 dynes/cm at 25°c. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation of the presence of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 What Is Responsible For The High Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Since these intermolecular forces vary. The surface tension of. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 17 “Water and Aqueous Systems” PowerPoint Presentation What Is Responsible For The High Surface Tension Of Water Since these intermolecular forces vary. Surface tension is a manifestation of the presence of. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).. What Is Responsible For The High Surface Tension Of Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a. What Is Responsible For The High Surface Tension Of Water.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Is Responsible For The High Surface Tension Of Water The surface tension of water is 72 dynes/cm at 25°c. Surface tension is a manifestation of the presence of. It would take a force of 72 dynes to break a surface film of water 1 cm long. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. The cohesive forces between liquid molecules are responsible. What Is Responsible For The High Surface Tension Of Water.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. Since these intermolecular forces vary. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation of the presence of. Surface tension is the energy, or work, required. What Is Responsible For The High Surface Tension Of Water.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with What Is Responsible For The High Surface Tension Of Water The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. Water has a surface tension of 0.07275 joule per square metre at. What Is Responsible For The High Surface Tension Of Water.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water is 72 dynes/cm at 25°c. Water has a surface. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Is Responsible For The High Surface Tension Of Water The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Next to mercury, water has the highest surface tension of all commonly occurring liquids.. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Is Responsible For The High Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water decreases significantly with. The surface tension of water is 72 dynes/cm at 25°c. Since these intermolecular forces vary. Surface tension is a manifestation of the presence of. Next to mercury, water has the highest surface. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Is Responsible For The High Surface Tension Of Water Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Since these intermolecular forces vary. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation of. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download What Is Responsible For The High Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. The surface tension of water decreases significantly with. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. What Is Responsible For The High Surface Tension Of Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Is Responsible For The High Surface Tension Of Water Next to mercury, water has the highest surface tension of all commonly occurring liquids. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1714027 What Is Responsible For The High Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is a manifestation of the presence of. The cohesive forces between liquid molecules are responsible for the. Next to mercury,. What Is Responsible For The High Surface Tension Of Water.
From byjus.com
Explain the surface tension phenomenon with examples. What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. The cohesive forces between liquid molecules are responsible for the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). It would take a force of 72 dynes to break a surface film of water 1 cm long. Next to mercury, water has the highest surface. What Is Responsible For The High Surface Tension Of Water.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… What Is Responsible For The High Surface Tension Of Water Since these intermolecular forces vary. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of. What Is Responsible For The High Surface Tension Of Water.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation What Is Responsible For The High Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension. What Is Responsible For The High Surface Tension Of Water.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts What Is Responsible For The High Surface Tension Of Water The surface tension of water decreases significantly with. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Since these intermolecular forces vary. Next to mercury, water has the highest surface tension of all commonly occurring liquids.. What Is Responsible For The High Surface Tension Of Water.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Is Responsible For The High Surface Tension Of Water Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is a manifestation of the presence of. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the. What Is Responsible For The High Surface Tension Of Water.