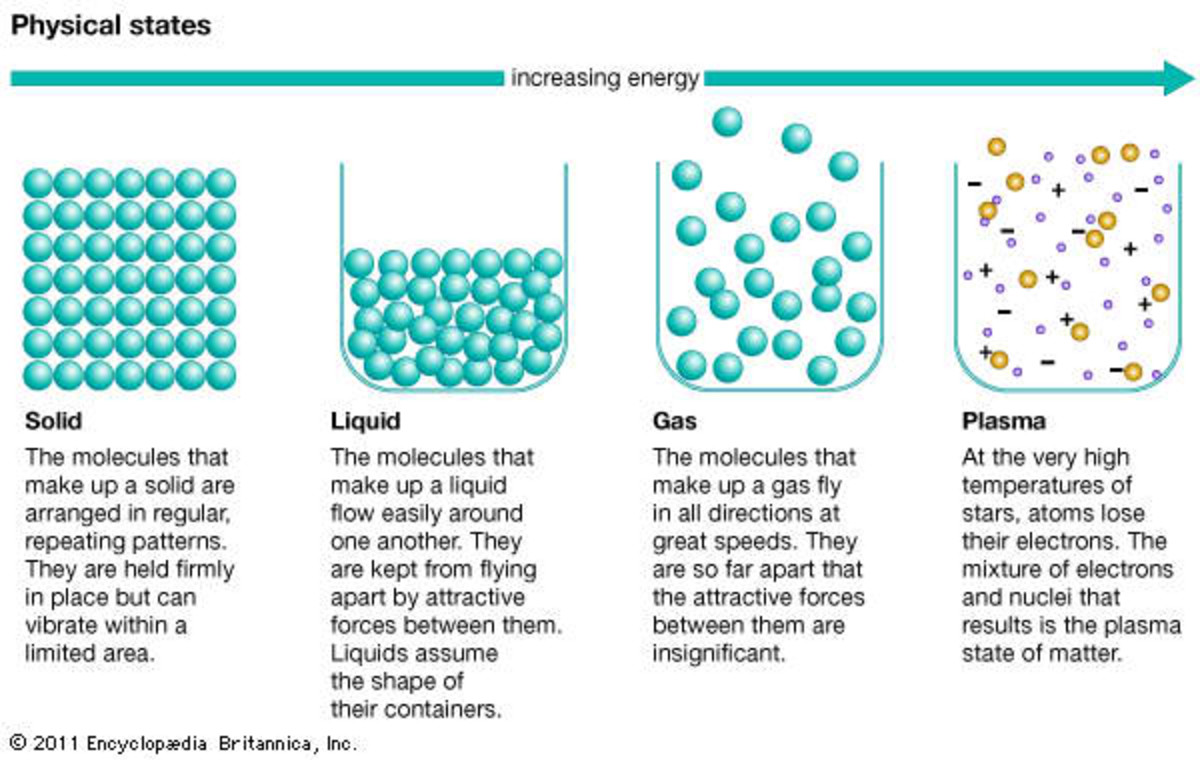
Solid Liquid Phase Called . the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. At high pressures and low temperatures, the substance is in the solid phase. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. matter undergoes phase changes or phase transitions from one state of matter to another. states of matter include solid, liquid or gas phases.
from owlcation.com
states of matter include solid, liquid or gas phases. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. At high pressures and low temperatures, the substance is in the solid phase. matter undergoes phase changes or phase transitions from one state of matter to another.
All About Matter An Introduction to the Basics Owlcation
Solid Liquid Phase Called matter undergoes phase changes or phase transitions from one state of matter to another. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. At high pressures and low temperatures, the substance is in the solid phase. matter undergoes phase changes or phase transitions from one state of matter to another. states of matter include solid, liquid or gas phases.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. matter undergoes phase changes or phase transitions from one state of matter to another. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. states of matter include solid, liquid or gas phases. the temperature. Solid Liquid Phase Called.
From lessonlibcerebellum.z21.web.core.windows.net
Matter Chart Solid Liquid Gas Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. At high pressures and low temperatures, the substance is in the solid phase. phase transition is when. Solid Liquid Phase Called.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid Liquid Phase Called matter undergoes phase changes or phase transitions from one state of matter to another. At high pressures and low temperatures, the substance is in the solid phase. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. the temperature at which the solid and liquid phases of a given. Solid Liquid Phase Called.
From spectrumnews1.com
The amount of water on Earth is a constant Solid Liquid Phase Called the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. matter undergoes phase changes or phase transitions from one state of matter to another. At high pressures. Solid Liquid Phase Called.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid Liquid Phase Called states of matter include solid, liquid or gas phases. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. matter undergoes phase changes or phase transitions from one state of matter to another. At high pressures and low temperatures, the substance is in the solid. Solid Liquid Phase Called.
From www.slideserve.com
PPT Solid Liquid Phase Diagrams PowerPoint Presentation, free Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. matter undergoes phase changes or phase transitions from one state of matter to another. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. At high. Solid Liquid Phase Called.
From rohanexmoses.blogspot.com
Solid to Gas is Called RohanexMoses Solid Liquid Phase Called phase transition is when a substance changes from a solid, liquid, or gas state to a different state. matter undergoes phase changes or phase transitions from one state of matter to another. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. Every element and. Solid Liquid Phase Called.
From newsela.com
There are three phases of matter solid, liquid and gas Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. states of matter. Solid Liquid Phase Called.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends. Solid Liquid Phase Called.
From courses.lumenlearning.com
Phase Changes Boundless Chemistry Solid Liquid Phase Called the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. At high pressures and low temperatures, the substance is in the solid phase.. Solid Liquid Phase Called.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. phase transition is when. Solid Liquid Phase Called.
From www.grc.nasa.gov
Phases of Matter Solid Liquid Phase Called the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. states of matter include solid, liquid or gas phases. Every element and substance can transition from one. Solid Liquid Phase Called.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. matter undergoes phase changes or phase transitions from one state of matter to another. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. states of matter include solid, liquid or gas. Solid Liquid Phase Called.
From kids.britannica.com
solid Students Britannica Kids Homework Help Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. states of matter include solid, liquid or gas phases. each distinct form is called a phase, but the concept of. Solid Liquid Phase Called.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid Liquid Phase Called matter undergoes phase changes or phase transitions from one state of matter to another. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. states. Solid Liquid Phase Called.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends. Solid Liquid Phase Called.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Liquid Phase Called states of matter include solid, liquid or gas phases. matter undergoes phase changes or phase transitions from one state of matter to another. At high pressures and low temperatures, the substance is in the solid phase. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point. Solid Liquid Phase Called.
From fr.dreamstime.com
Illustration Pour Des Changements D'état Entre Solide, Le Liquide Et Le Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. states of matter include solid, liquid or gas phases. phase transition is when a substance changes from a solid, liquid, or gas. Solid Liquid Phase Called.
From unistudium.unipg.it
Phase Diagrams Solid Liquid Phase Called phase transition is when a substance changes from a solid, liquid, or gas state to a different state. states of matter include solid, liquid or gas phases. matter undergoes phase changes or phase transitions from one state of matter to another. Every element and substance can transition from one phase to another at a specific combination of. Solid Liquid Phase Called.
From www.slideserve.com
PPT Solid Liquid Phase Diagrams PowerPoint Presentation, free Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. matter undergoes phase changes or phase transitions from one state of matter to another. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. At high. Solid Liquid Phase Called.
From www.theschoolrun.com
What are states of matter? TheSchoolRun Solid Liquid Phase Called states of matter include solid, liquid or gas phases. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. each distinct form is called a phase,. Solid Liquid Phase Called.
From learningnadeaurankers.z21.web.core.windows.net
Matter Chart Solid Liquid Gas Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. states of matter include solid, liquid or gas. Solid Liquid Phase Called.
From giogyfrmx.blob.core.windows.net
Deposition Phases Of Matter at Jennifer Comeaux blog Solid Liquid Phase Called each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. states of matter include solid, liquid or gas phases. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance. Solid Liquid Phase Called.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation Solid Liquid Phase Called states of matter include solid, liquid or gas phases. matter undergoes phase changes or phase transitions from one state of matter to another. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. each distinct form is called a phase, but the concept of phase defined as a. Solid Liquid Phase Called.
From stock.adobe.com
states of matter solids liquids and gases. Matter appears in three Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. the temperature at which the solid and liquid phases of a given substance are in equilibrium. Solid Liquid Phase Called.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. states of matter include solid, liquid or gas. Solid Liquid Phase Called.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID994333 Solid Liquid Phase Called phase transition is when a substance changes from a solid, liquid, or gas state to a different state. states of matter include solid, liquid or gas phases. matter undergoes phase changes or phase transitions from one state of matter to another. Every element and substance can transition from one phase to another at a specific combination of. Solid Liquid Phase Called.
From partdiagramaminabakery5v.z14.web.core.windows.net
What Is The Critical Point On A Phase Diagram Solid Liquid Phase Called matter undergoes phase changes or phase transitions from one state of matter to another. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends. Solid Liquid Phase Called.
From fyoecbagh.blob.core.windows.net
Conversion Of Liquid Into Solid Is Called at Roger Feliciano blog Solid Liquid Phase Called the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. At high pressures and low temperatures, the substance is in the solid phase. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can. Solid Liquid Phase Called.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID3091525 Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion of a system, extends beyond a. phase transition is when a substance changes from a solid, liquid, or gas state to a. Solid Liquid Phase Called.
From www.britannica.com
phase Definition & Facts Britannica Solid Liquid Phase Called phase transition is when a substance changes from a solid, liquid, or gas state to a different state. At high pressures and low temperatures, the substance is in the solid phase. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. each distinct form is. Solid Liquid Phase Called.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. matter undergoes phase changes or phase transitions from one state of matter to another. states of matter include solid, liquid or gas phases. Every element and. Solid Liquid Phase Called.
From learncheme.com
solidsolidliquidphasediagramsconceptests2 LearnChemE Solid Liquid Phase Called Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. states of matter include solid, liquid or gas phases. the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of. matter undergoes phase changes or phase transitions. Solid Liquid Phase Called.
From hxenwilps.blob.core.windows.net
SolidLiquid Phase Change Phenomena at Bobbie ODonnell blog Solid Liquid Phase Called matter undergoes phase changes or phase transitions from one state of matter to another. At high pressures and low temperatures, the substance is in the solid phase. states of matter include solid, liquid or gas phases. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition. Solid Liquid Phase Called.
From sciencenotes.org
States of Matter Solid Liquid Phase Called At high pressures and low temperatures, the substance is in the solid phase. states of matter include solid, liquid or gas phases. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. each distinct form is called a phase, but the concept of phase defined as a homogeneous portion. Solid Liquid Phase Called.