Calorimetry Equation Specific Heat . the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. (recall that the temperature change δt δ t is the. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). Calculate and interpret heat and related properties using typical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). The specific heat is numerically equal to. Explain the technique of calorimetry.
from www.youtube.com
q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). The specific heat is numerically equal to. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. (recall that the temperature change δt δ t is the. Explain the technique of calorimetry. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calculate and interpret heat and related properties using typical.
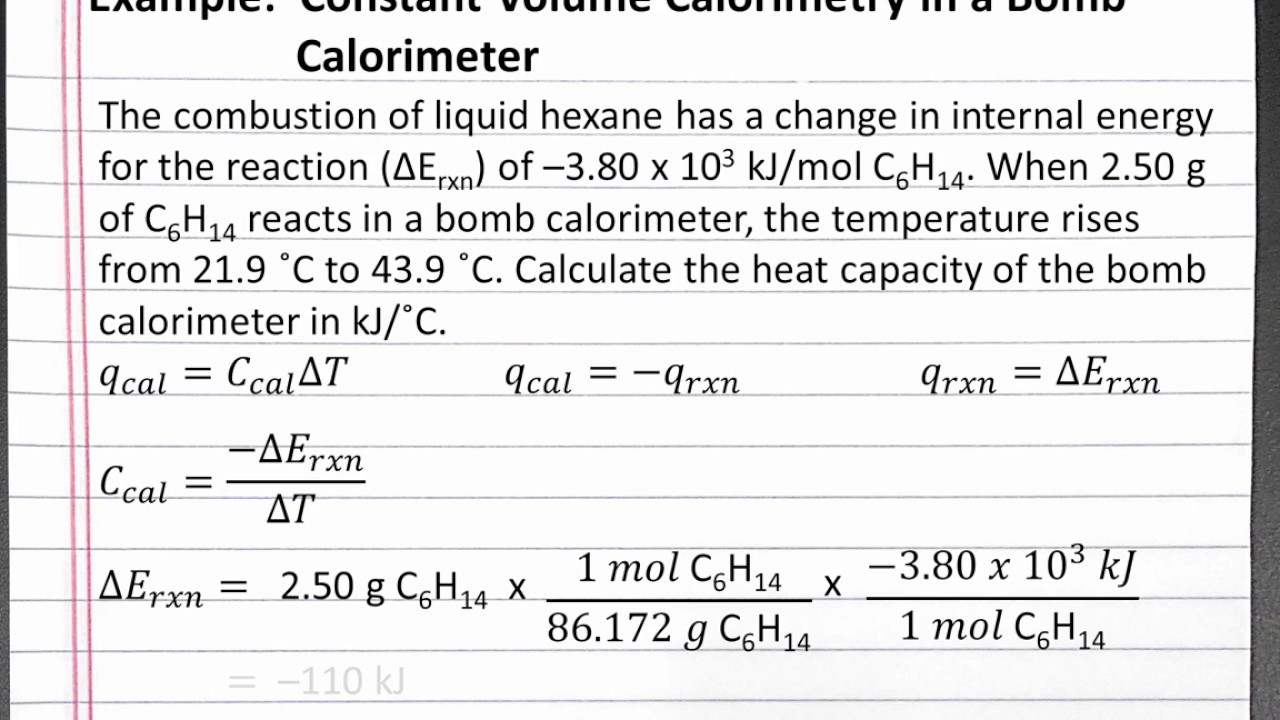
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube
Calorimetry Equation Specific Heat the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. Explain the technique of calorimetry. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. (recall that the temperature change δt δ t is the. Calculate and interpret heat and related properties using typical. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). The specific heat is numerically equal to. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p).
From classmediaregrouping.z14.web.core.windows.net
Calculating Specific Heat Examples Calorimetry Equation Specific Heat the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. Calculate and interpret heat and related properties using typical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance,. Calorimetry Equation Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Equation Specific Heat The specific heat is numerically equal to. (recall that the temperature change δt δ t is the. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). the specific heat (c s) of a substance is the amount of energy needed. Calorimetry Equation Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Equation Specific Heat the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the si. Calorimetry Equation Specific Heat.
From gioxxobze.blob.core.windows.net
Calorimetry Formula Reaction at Mazie Miller blog Calorimetry Equation Specific Heat Calculate and interpret heat and related properties using typical. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. (recall that the temperature change δt δ t is the. The specific heat is numerically equal to. the symbol c stands. Calorimetry Equation Specific Heat.
From exywsebyp.blob.core.windows.net
Calorimetry Equation Physics at Charles Marshall blog Calorimetry Equation Specific Heat the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). Explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the. Calorimetry Equation Specific Heat.
From www.youtube.com
Specific Heat Solving for the Final Temperature YouTube Calorimetry Equation Specific Heat Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. (recall that the temperature change δt δ t is the. the symbol c stands for the specific. Calorimetry Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Equation Specific Heat Calculate and interpret heat and related properties using typical. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance,. Calorimetry Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Equation Specific Heat The specific heat is numerically equal to. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. the specific heat. Calorimetry Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Equation Specific Heat Calculate and interpret heat and related properties using typical. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. Explain the technique of calorimetry. the si unit for specific heat is j /(kg× k) j / (k g × k). Calorimetry Equation Specific Heat.
From quizzlibhofmann.z19.web.core.windows.net
Specific Heat Calculation Examples Calorimetry Equation Specific Heat The specific heat is numerically equal to. Calculate and interpret heat and related properties using typical. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the specific heat capacity (\ (c\)) of a substance,. Calorimetry Equation Specific Heat.
From giojclhhk.blob.core.windows.net
How To Calculate Calorimeter Constant Problems at Michael Massie blog Calorimetry Equation Specific Heat Explain the technique of calorimetry. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). the specific heat (c s). Calorimetry Equation Specific Heat.
From www.youtube.com
Specific heat capacity YouTube Calorimetry Equation Specific Heat the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the specific heat (c s) of a substance is the. Calorimetry Equation Specific Heat.
From exywsebyp.blob.core.windows.net
Calorimetry Equation Physics at Charles Marshall blog Calorimetry Equation Specific Heat the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). (recall that the temperature change δt δ t is the. The specific heat is numerically equal to. the si unit for specific heat is j. Calorimetry Equation Specific Heat.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Equation Specific Heat Explain the technique of calorimetry. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase.. Calorimetry Equation Specific Heat.
From www.youtube.com
Calorimetry Calculate Specific Heat YouTube Calorimetry Equation Specific Heat the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is. Calorimetry Equation Specific Heat.
From exyazvuru.blob.core.windows.net
Bomb Calorimeter Formula Heat at Filiberto Darnell blog Calorimetry Equation Specific Heat Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the specific heat (c s) of a substance is the amount of energy needed to raise the. Calorimetry Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Equation Specific Heat (recall that the temperature change δt δ t is the. The specific heat is numerically equal to. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). the si unit for specific heat is j. Calorimetry Equation Specific Heat.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimetry Equation Specific Heat the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. The specific heat is numerically equal to. q =. Calorimetry Equation Specific Heat.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Calorimetry Equation Specific Heat the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). Calculate and interpret. Calorimetry Equation Specific Heat.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Equation Specific Heat Explain the technique of calorimetry. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1. Calorimetry Equation Specific Heat.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Equation Specific Heat the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on. Calorimetry Equation Specific Heat.
From www.tessshebaylo.com
What Is The Equation To Solve For Amount Of Heat Energy Tessshebaylo Calorimetry Equation Specific Heat (recall that the temperature change δt δ t is the. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of.. Calorimetry Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Equation Specific Heat the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p). Explain the technique of calorimetry. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c). Calorimetry Equation Specific Heat.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Calorimetry Equation Specific Heat the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. (recall that the temperature change δt δ t is the. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1. Calorimetry Equation Specific Heat.
From fyobkyyye.blob.core.windows.net
Calorimetry Equation Q at Billy Hudson blog Calorimetry Equation Specific Heat q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. The specific heat is numerically equal to. Calculate and interpret heat and related properties using typical. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is. Calorimetry Equation Specific Heat.
From classmediaregrouping.z14.web.core.windows.net
Specific Heat Calculation Formula Calorimetry Equation Specific Heat Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. The specific heat is numerically equal to. the specific heat (c s) of a substance is. Calorimetry Equation Specific Heat.
From www.tessshebaylo.com
Calorimeter Equation Final Temperature Tessshebaylo Calorimetry Equation Specific Heat Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry. the specific heat capacity (\ (c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is. Calorimetry Equation Specific Heat.
From www.youtube.com
Specific heat capacity of solid by the method of mixture YouTube Calorimetry Equation Specific Heat the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. Explain the technique of calorimetry. the si unit for specific. Calorimetry Equation Specific Heat.
From users.highland.edu
Calorimetry Calorimetry Equation Specific Heat Calculate and interpret heat and related properties using typical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. (recall that. Calorimetry Equation Specific Heat.
From saylordotorg.github.io
Calorimetry Calorimetry Equation Specific Heat the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). The specific heat is numerically equal to. Explain the technique of calorimetry. the specific heat (c s) of a substance is the amount of energy needed to raise the temperature of. Calorimetry Equation Specific Heat.
From exykexyqb.blob.core.windows.net
Bomb Of Calorimetry at Jeanne Gee blog Calorimetry Equation Specific Heat The specific heat is numerically equal to. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. the specific heat (c s) of a substance is the. Calorimetry Equation Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Equation Specific Heat The specific heat is numerically equal to. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. q = mcδt,. Calorimetry Equation Specific Heat.
From en.ppt-online.org
What is enthalpy? online presentation Calorimetry Equation Specific Heat Explain the technique of calorimetry. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). (recall that the temperature change δt. Calorimetry Equation Specific Heat.
From www.youtube.com
Specific Heat Equation Stated Clearly YouTube Calorimetry Equation Specific Heat The specific heat is numerically equal to. (recall that the temperature change δt δ t is the. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. the si unit for specific heat is j /(kg× k) j / (k g. Calorimetry Equation Specific Heat.
From dxofdddzc.blob.core.windows.net
Calorimeter Heat Capacity Equation at Denise Chavez blog Calorimetry Equation Specific Heat the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. the si unit for specific heat is j /(kg× k) j / (k g × k) or j /(kg×∘ c) j / (k g × ∘ c). q = mcδt, where q is the symbol for heat. Calorimetry Equation Specific Heat.