Calorimeter Viva Questions . When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the calorimeter. A calorimeter is an apparatus used for calculating the heat. We do this using simple calorimetry. Calorimetry is the measurement enthalpy changes in chemical reactions. We can experimentally determine the relative amounts of energy released by a fuel. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. 1) what is a calorimeter? Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Important calorimeter questions with answers. A simple calorimeter can be made from a polystyrene drinking cup, a.
from studylib.net
We do this using simple calorimetry. If the heat capacity of the calorimeter. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. 1) what is a calorimeter? When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Calorimetry is the measurement enthalpy changes in chemical reactions. A simple calorimeter can be made from a polystyrene drinking cup, a. A calorimeter is an apparatus used for calculating the heat. We can experimentally determine the relative amounts of energy released by a fuel.
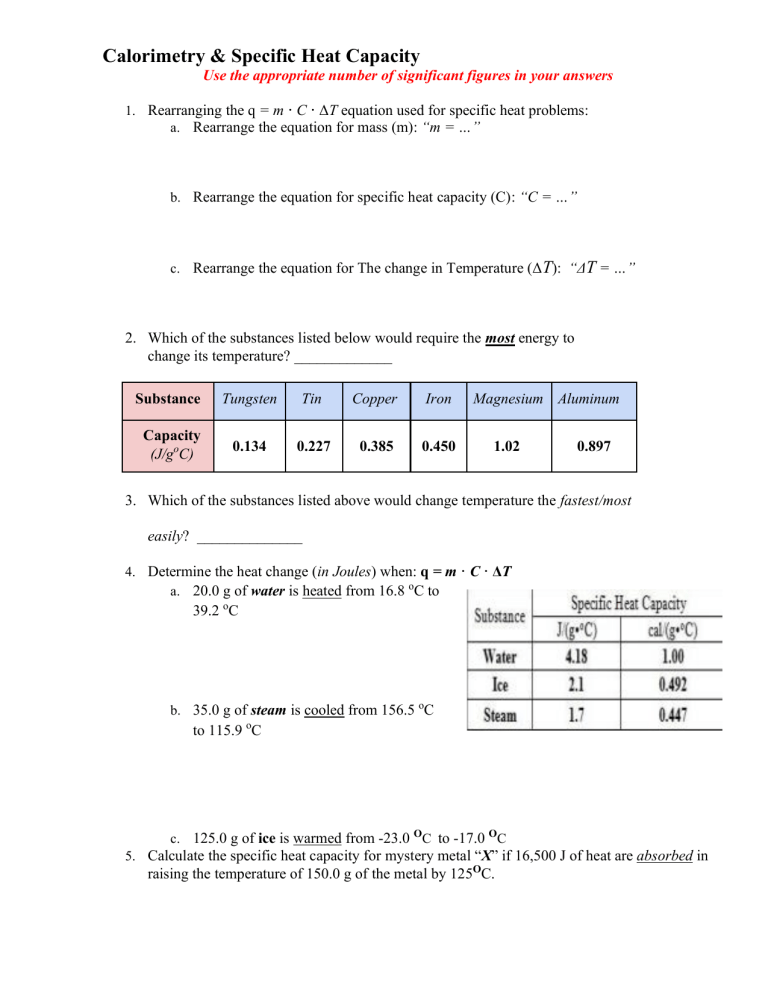
Calorimetry Worksheet
Calorimeter Viva Questions If the heat capacity of the calorimeter. Calorimetry is the measurement enthalpy changes in chemical reactions. We do this using simple calorimetry. A calorimeter is an apparatus used for calculating the heat. We can experimentally determine the relative amounts of energy released by a fuel. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. 1) what is a calorimeter? A simple calorimeter can be made from a polystyrene drinking cup, a. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. If the heat capacity of the calorimeter. Important calorimeter questions with answers.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Viva Questions 1) what is a calorimeter? Calorimetry is the measurement enthalpy changes in chemical reactions. A simple calorimeter can be made from a polystyrene drinking cup, a. If the heat capacity of the calorimeter. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. In this set of practice. Calorimeter Viva Questions.
From www.scribd.com
Questions Calorimetry Using A Bomb Calorimeter PDF Power Station Calorimeter Viva Questions We do this using simple calorimetry. A simple calorimeter can be made from a polystyrene drinking cup, a. We can experimentally determine the relative amounts of energy released by a fuel. 1) what is a calorimeter? Important calorimeter questions with answers. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate. Calorimeter Viva Questions.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Viva Questions We do this using simple calorimetry. Important calorimeter questions with answers. We can experimentally determine the relative amounts of energy released by a fuel. If the heat capacity of the calorimeter. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. In this set of practice questions, we. Calorimeter Viva Questions.
From www.iitianacademy.com
AP Chemistry 6.4 Heat Capacity and Calorimetry Exam Style questions Calorimeter Viva Questions Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calorimetry is the measurement enthalpy changes in chemical reactions. 1) what is a calorimeter? When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. In this set of practice questions, we. Calorimeter Viva Questions.
From studylib.net
Bomb Calorimeter Questions Calorimeter Viva Questions Calorimetry is the measurement enthalpy changes in chemical reactions. 1) what is a calorimeter? In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Important calorimeter questions with answers. We can experimentally determine the relative amounts of energy released by a fuel. If the heat. Calorimeter Viva Questions.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimeter Viva Questions 1) what is a calorimeter? We can experimentally determine the relative amounts of energy released by a fuel. Important calorimeter questions with answers. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. Calorimeter Viva Questions.
From losform.athensmutualaid.net
Student Exploration Calorimetry Lab Gizmo Answer Key losformathens Calorimeter Viva Questions 1) what is a calorimeter? When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. A calorimeter is an apparatus used for calculating the heat. A simple calorimeter can be made from a polystyrene drinking cup, a. We do this using simple calorimetry. Use our revision notes on calorimetry for a level chemistry. Calorimeter Viva Questions.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Calorimeter Viva Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the calorimeter. A calorimeter is an apparatus used for calculating the heat. Calorimetry. Calorimeter Viva Questions.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimeter Viva Questions We can experimentally determine the relative amounts of energy released by a fuel. Important calorimeter questions with answers. 1) what is a calorimeter? A simple calorimeter can be made from a polystyrene drinking cup, a. We do this using simple calorimetry. A calorimeter is an apparatus used for calculating the heat. In this set of practice questions, we will go. Calorimeter Viva Questions.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimeter Viva Questions A simple calorimeter can be made from a polystyrene drinking cup, a. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. We do this using simple calorimetry.. Calorimeter Viva Questions.
From www.studocu.com
Calorimetry Gizmo Student Exploration Calorimetry Lab Gizmo Warmup Calorimeter Viva Questions When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. 1) what is a calorimeter? Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. We can experimentally determine the relative amounts of energy released by a fuel. In this set. Calorimeter Viva Questions.
From byjus.com
What is bomb calorimeter? Calorimeter Viva Questions We do this using simple calorimetry. 1) what is a calorimeter? Important calorimeter questions with answers. Calorimetry is the measurement enthalpy changes in chemical reactions. A calorimeter is an apparatus used for calculating the heat. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,.. Calorimeter Viva Questions.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimeter Viva Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Important calorimeter questions with answers. We do this using simple calorimetry. A calorimeter. Calorimeter Viva Questions.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Viva Questions Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. We do this using simple calorimetry. A simple calorimeter can be made from a polystyrene drinking cup, a. 1) what is. Calorimeter Viva Questions.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimeter Viva Questions When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. A calorimeter is an apparatus used for calculating the heat. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. We do this using simple calorimetry. A simple calorimeter can be. Calorimeter Viva Questions.
From studylib.net
Calorimetry Calorimeter Viva Questions A calorimeter is an apparatus used for calculating the heat. A simple calorimeter can be made from a polystyrene drinking cup, a. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. We can experimentally determine the relative amounts of energy released by a fuel. We do this. Calorimeter Viva Questions.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Calorimeter Viva Questions Important calorimeter questions with answers. We do this using simple calorimetry. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. 1) what. Calorimeter Viva Questions.
From www.slideserve.com
PPT Heat of Reaction Experiment 23 PowerPoint Presentation, free Calorimeter Viva Questions 1) what is a calorimeter? A calorimeter is an apparatus used for calculating the heat. We can experimentally determine the relative amounts of energy released by a fuel. Important calorimeter questions with answers. We do this using simple calorimetry. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. Calorimeter Viva Questions.
From www.youtube.com
Bomb Calorimeter & Tricks to solve Bomb Calorimeter questions easily Calorimeter Viva Questions Calorimetry is the measurement enthalpy changes in chemical reactions. Important calorimeter questions with answers. A calorimeter is an apparatus used for calculating the heat. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. 1) what is a calorimeter? In this set of practice questions, we will go. Calorimeter Viva Questions.
From materialdbtracy.z13.web.core.windows.net
Calorimetry Worksheet With Solutions Calorimeter Viva Questions If the heat capacity of the calorimeter. Important calorimeter questions with answers. We do this using simple calorimetry. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. 1) what is a calorimeter? We can experimentally determine the relative amounts of energy released by a. Calorimeter Viva Questions.
From www.studocu.com
Calorimetry and Specific Heat Lab Question Using a calorimeter, how Calorimeter Viva Questions A calorimeter is an apparatus used for calculating the heat. 1) what is a calorimeter? A simple calorimeter can be made from a polystyrene drinking cup, a. We do this using simple calorimetry. Important calorimeter questions with answers. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity.. Calorimeter Viva Questions.
From www.studocu.com
Copy of Optional Gizmo Calorimetry Student Exploration Calorimetry Calorimeter Viva Questions We can experimentally determine the relative amounts of energy released by a fuel. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. A calorimeter is an apparatus used for calculating. Calorimeter Viva Questions.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Viva Questions Important calorimeter questions with answers. We can experimentally determine the relative amounts of energy released by a fuel. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. If the heat capacity of the calorimeter. Use our revision notes on calorimetry for a level chemistry. Calorimeter Viva Questions.
From studylib.net
Calorimetry Worksheet Calorimeter Viva Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. If the heat capacity of the calorimeter. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. A simple calorimeter can be made from a polystyrene drinking cup,. Calorimeter Viva Questions.
From www.youtube.com
Graph questions from calorimetry (numericals), "Give reason"type Calorimeter Viva Questions When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Important calorimeter questions with answers. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. If the heat capacity of the calorimeter. We can experimentally determine the relative amounts of energy. Calorimeter Viva Questions.
From studylib.net
Calorimetry Worksheet Calorimeter Viva Questions A calorimeter is an apparatus used for calculating the heat. If the heat capacity of the calorimeter. We can experimentally determine the relative amounts of energy released by a fuel. 1) what is a calorimeter? When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Calorimetry is the measurement enthalpy changes in chemical. Calorimeter Viva Questions.
From worksheetmediadwaum.z14.web.core.windows.net
Chemistry Calorimetry Problems 1 Answers Calorimeter Viva Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. 1) what is a calorimeter? A simple calorimeter can be made from a polystyrene drinking cup, a. Important calorimeter questions with answers. When 1.00 g of coal is burned in a bomb calorimeter, the temperature. Calorimeter Viva Questions.
From www.youtube.com
Thermal Properties of Matter (Calorimetry, Calorimeter) part 3 Calorimeter Viva Questions We do this using simple calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. A calorimeter is an apparatus used for calculating the heat. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. 1) what is a calorimeter? If. Calorimeter Viva Questions.
From www.coursehero.com
[Solved] Calorimetry Questions I set up a calorimetry experiment as Calorimeter Viva Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Calorimetry is the measurement enthalpy changes in chemical reactions. A calorimeter is an apparatus used for calculating the heat. A simple calorimeter can be made from a polystyrene drinking cup, a. If the heat capacity. Calorimeter Viva Questions.
From www.bartleby.com
Answered Data for Calorimetry Part A Mass of… bartleby Calorimeter Viva Questions We do this using simple calorimetry. A simple calorimeter can be made from a polystyrene drinking cup, a. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,.. Calorimeter Viva Questions.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Viva Questions We can experimentally determine the relative amounts of energy released by a fuel. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. We do this using simple calorimetry. A simple calorimeter can be made from a polystyrene drinking cup, a. Use our revision notes on calorimetry for a level chemistry to understand. Calorimeter Viva Questions.
From www.science-revision.co.uk
Calorimetry Calorimeter Viva Questions If the heat capacity of the calorimeter. Important calorimeter questions with answers. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. A simple calorimeter can be made from a polystyrene drinking cup, a. We can experimentally determine the relative amounts of energy released by. Calorimeter Viva Questions.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Viva Questions When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. We do this using simple calorimetry. Calorimetry is the measurement enthalpy changes in chemical reactions. 1) what is a calorimeter? A simple calorimeter can be made from a polystyrene drinking cup, a. We can experimentally determine the relative amounts of energy released by. Calorimeter Viva Questions.
From www.youtube.com
CALORIMETRY Calorimetry Class 10 ICSE Calorimetry Theory and Calorimeter Viva Questions Important calorimeter questions with answers. We can experimentally determine the relative amounts of energy released by a fuel. A calorimeter is an apparatus used for calculating the heat. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. If the heat capacity of the calorimeter. We do this. Calorimeter Viva Questions.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Viva Questions We do this using simple calorimetry. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Important calorimeter questions with answers. We can experimentally determine the relative amounts of energy released by a fuel. In this set of practice questions, we will go over the main types of. Calorimeter Viva Questions.