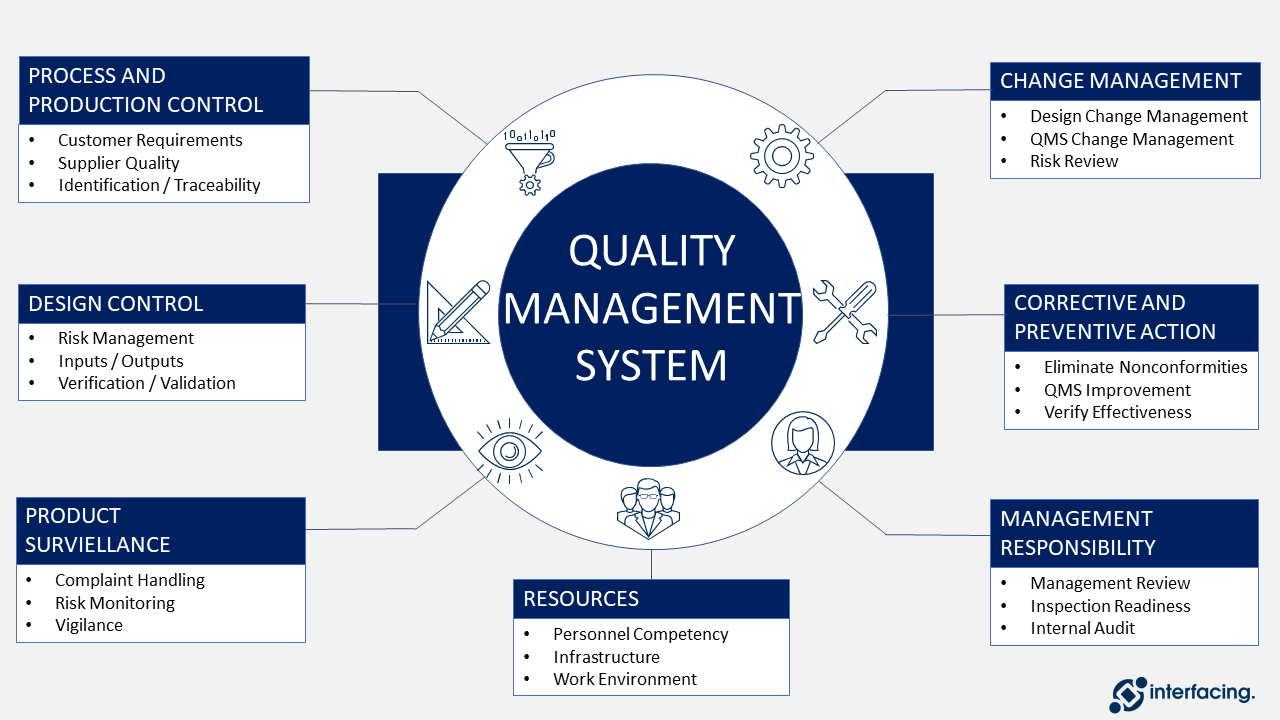
Medical Device Company Quality Policy . In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be.
from quoteshomede.blogspot.com
The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. In this article, we will.
Medical Device Quality Management System Quotes Home
Medical Device Company Quality Policy If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. In this article, we will.
From cellmed.co.zw
Quality Policy CellMed Health Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. The fda takes enforcement action to bring medical device manufacturers into. Medical Device Company Quality Policy.
From gvrp.in
The National Medical Devices Policy 2023 Everything you need to know GVRP Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. In this article, we. Medical Device Company Quality Policy.
From www.exsolutiongroup.com
ISO 134852003 Quality Management System for Medical Devices QMS for Medical Devices Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet. Medical Device Company Quality Policy.
From hardcoreqms.com
Building a Quality Policy for ISO 13485 (with examples) Medical Device Company Quality Policy If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. In this article, we will. Tmc’s quality system is specifically intended for the design, development,. Medical Device Company Quality Policy.
From www.aplyon.com
Medical Device Quality Agreement US Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your. Medical Device Company Quality Policy.
From www.darvoni.com
Quality Policy Darvoni Beacon Medicare Limited Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. Medical Device Company Quality Policy.
From www.orthopaedic-implants.com
Quality Policy Medical Device Company Quality Policy If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical. Medical Device Company Quality Policy.
From quoteshomede.blogspot.com
Medical Device Quality Management System Quotes Home Medical Device Company Quality Policy In this article, we will. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. The fda takes enforcement action to bring medical device manufacturers. Medical Device Company Quality Policy.
From www.qmdocs.com
Medical Device Design And Development Plan QMDocs Quality Management System Templates Medical Device Company Quality Policy In this article, we will. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Device Company Quality Policy.
From www.greenlight.guru
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Devices Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not. Medical Device Company Quality Policy.
From www.mastercontrol.com
Medical Device Quality Management Ultimate Guide MasterControl Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your company, you’ve come. Medical Device Company Quality Policy.
From easymedicaldevice.com
About Us Medical Device Regulation and ISO quality standard Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure. Medical Device Company Quality Policy.
From iziel.com
QMS Documentation for Medical Devices ISO 13485 Certification IZiel Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet. Medical Device Company Quality Policy.
From www.impactqa.com
Why QA & QC are an essential part of Medical Device Testing? Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your company, you’ve come to the. Medical Device Company Quality Policy.
From www.joharidigital.com
Importance of Quality Management System in Medical Device Manufacturing Medical Device Johari Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Device Company Quality Policy.
From microcathco.com
Quality Policy framed Microcatheter Components Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Device Company Quality Policy.
From www.cognidox.com
4 ways to build a medical device quality management system Medical Device Company Quality Policy Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. In this article, we will. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution. Medical Device Company Quality Policy.
From intellisoft.io
Medical Device QMS What Is It And How To Build a QMS Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Device Company Quality Policy.
From www.aplyon.com
Medical Device Quality Agreement US Medical Device Company Quality Policy Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. If you are wondering how to make an excellent quality policy for your company, you’ve come to the. Medical Device Company Quality Policy.
From www.freyrsolutions.com
Quality Management System (QMS) in Medical Devices Freyr Global Regulatory Solutions and Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet. Medical Device Company Quality Policy.
From www.qualio.com
Medical device quality management system template 8 powerful options Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. In this article, we will. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Device Company Quality Policy.
From www.template.net
Medical Device Quality Agreement Template Google Docs, Word, Apple Pages Medical Device Company Quality Policy If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. Tmc’s quality system is specifically intended for the design, development,. Medical Device Company Quality Policy.
From www.genlite.in
Quality Policy & Objectives Genlite Medical Device Company Quality Policy In this article, we will. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Device Company Quality Policy.
From www.slideserve.com
PPT Medical Device Quality Enhancement An Industry Perspective PowerPoint Presentation ID Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices. Medical Device Company Quality Policy.
From www.greenlight.guru
Quality Assurance vs. Quality Control in the Medical Device Industry Medical Device Company Quality Policy In this article, we will. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right. Medical Device Company Quality Policy.
From www.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Device QMS Requirements Medical Device Company Quality Policy In this article, we will. If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. Medical Device Company Quality Policy.
From soptemplates.org
Quality Policy Statement for Medical Devices Medical Device Company Quality Policy Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. The fda takes enforcement action to bring medical device manufacturers into. Medical Device Company Quality Policy.
From www.youtube.com
European Medical Device Registration Chapter 3 Quality Management System YouTube Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your company, you’ve come to the. Medical Device Company Quality Policy.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Regulations to Know Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your. Medical Device Company Quality Policy.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Company Quality Policy If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. Tmc’s quality system is specifically intended for the design, development,. Medical Device Company Quality Policy.
From www.dotcompliance.com
The Essential Guide to ISO 14971 Dot Compliance Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. In this article, we will. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution. Medical Device Company Quality Policy.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Company Quality Policy If you are wondering how to make an excellent quality policy for your company, you’ve come to the right place. In this article, we will. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical. Medical Device Company Quality Policy.
From www.dochub.com
Medical device quality agreement template Fill out & sign online DocHub Medical Device Company Quality Policy In this article, we will. The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. If you are wondering how to make an excellent quality policy for your. Medical Device Company Quality Policy.
From blog.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Device QMS Requirements Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet regulatory. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the. Medical Device Company Quality Policy.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance Medical Device Company Quality Policy The fda takes enforcement action to bring medical device manufacturers into compliance when manufacturers may not be. Tmc’s quality system is specifically intended for the design, development, manufacture, and distribution of medical devices and the quality. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are safe, effective, and meet. Medical Device Company Quality Policy.