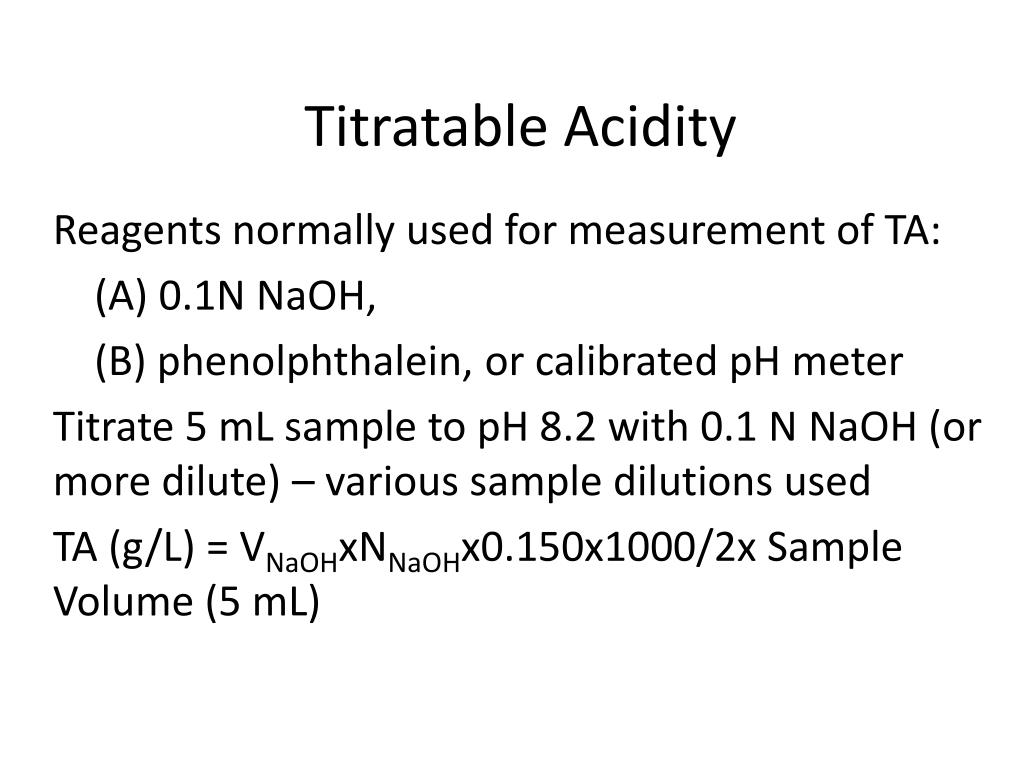
Titration Acidity Formula . Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The volume of the titrant added. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The acid content impacts the taste, color, and microbial stability. Acids, ta analysis is one of the most basic analyses in a winery lab. To evaluate the relationship between a titration’s equivalence point and its end point we need to.
from www.slideserve.com
Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The acid content impacts the taste, color, and microbial stability. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The volume of the titrant added. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. Acids, ta analysis is one of the most basic analyses in a winery lab. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes.
PPT FERMENTABLE NITROGEN PowerPoint Presentation, free download ID37175
Titration Acidity Formula Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The example below demonstrates the. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Acids, ta analysis is one of the most basic analyses in a winery lab. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The acid content impacts the taste, color, and microbial stability. The volume of the titrant added. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry Titration Acidity Formula The volume of the titrant added. The acid content impacts the taste, color, and microbial stability. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Technically, titratable acidity is. Titration Acidity Formula.
From chemistry.stackexchange.com
homework How to calculate the dissociation constant of a weak acid from the titration with a Titration Acidity Formula Acids, ta analysis is one of the most basic analyses in a winery lab. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The acid content impacts the taste, color, and. Titration Acidity Formula.
From www.chembuddy.com
Acid Base Titration and Equilibria Titration Acidity Formula The example below demonstrates the. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The volume of the titrant added. The above equation works only for neutralizations in which there is. Titration Acidity Formula.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Acidity Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. To. Titration Acidity Formula.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Acidity Formula Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Acids, ta analysis is one of the most basic analyses in a winery lab. The volume of the titrant added. The acid. Titration Acidity Formula.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Titration Acidity Formula The example below demonstrates the. The acid content impacts the taste, color, and microbial stability. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. To evaluate the relationship between a titration’s equivalence point and its end point we need to. Technically, titratable acidity is the sum of all of. Titration Acidity Formula.
From mungfali.com
Acid Base Titration Formula Titration Acidity Formula Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Acids, ta analysis is one of the most basic analyses in a winery lab. The volume of the titrant added. The example. Titration Acidity Formula.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Acidity Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The volume of the titrant added. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. Calculating the ta depends on the sample size you titrate and the concentration of sodium. Titration Acidity Formula.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration Acidity Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The volume of the titrant added. The acid content impacts the taste, color, and microbial stability. The. Titration Acidity Formula.
From mungfali.com
Acid Base Titration Formula Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The example below demonstrates the. The volume of the titrant added. Acids, ta analysis is one of the most basic analyses in a winery lab.. Titration Acidity Formula.
From mungfali.com
Acid Base Titration Calculation Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. Acids, ta analysis is one of the most basic analyses in a winery lab. The volume of the titrant added. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The acid content impacts the. Titration Acidity Formula.
From www.showme.com
Acidbase titration calculations Science, Chemistry, Acids and Bases ShowMe Titration Acidity Formula The example below demonstrates the. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The acid content impacts the taste, color, and microbial stability. The volume of the titrant added. Technically,. Titration Acidity Formula.
From www.youtube.com
Find out acidity of water by titration method find water acidity find water acidity by Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. The example below demonstrates the. The acid content impacts the taste, color, and microbial stability. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The following example exercise demonstrates the computation of ph for a. Titration Acidity Formula.
From www.youtube.com
Acid Base Titration Calculation Example YouTube Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Acids, ta analysis is one. Titration Acidity Formula.
From www.slideserve.com
PPT FERMENTABLE NITROGEN PowerPoint Presentation, free download ID37175 Titration Acidity Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The volume of the titrant added. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and. Titration Acidity Formula.
From mungfali.com
Acid Base Titration Formula Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. The acid content impacts the taste, color, and microbial stability. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Acids, ta analysis is one of the most basic analyses in a winery lab.. Titration Acidity Formula.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Technically, titratable acidity is. Titration Acidity Formula.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The example below demonstrates the. Acids, ta analysis is one of the most basic analyses in a winery lab. The following example exercise demonstrates the computation. Titration Acidity Formula.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID5872372 Titration Acidity Formula Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The acid content impacts the. Titration Acidity Formula.
From mavink.com
Acid Base Titration Equation Titration Acidity Formula Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The acid content impacts the taste, color, and microbial stability. The volume of the titrant added. Calculating the ta depends on the sample size you. Titration Acidity Formula.
From acid-base-titration.blogspot.com
Acid Base Titration Titrating Weak Acid With Strong Base Titration Acidity Formula The volume of the titrant added. The acid content impacts the taste, color, and microbial stability. The example below demonstrates the. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution.. Titration Acidity Formula.
From www.youtube.com
Titration of a weak base with a strong acid Chemistry Khan Academy YouTube Titration Acidity Formula Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The volume. Titration Acidity Formula.
From mungfali.com
Acid Alkali Titration Titration Acidity Formula The example below demonstrates the. The acid content impacts the taste, color, and microbial stability. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Acids, ta analysis is one of the most basic analyses in a winery lab. The above equation works only for neutralizations in which there is. Titration Acidity Formula.
From mungfali.com
Acid Base Titration Calculation Titration Acidity Formula Acids, ta analysis is one of the most basic analyses in a winery lab. The volume of the titrant added. The acid content impacts the taste, color, and microbial stability. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The above equation works only for neutralizations in which there is a 1:1 ratio. Titration Acidity Formula.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution YouTube Titration Acidity Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The example below demonstrates. Titration Acidity Formula.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Acidity Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. To evaluate the relationship between a titration’s equivalence point and its end point we need to. Calculating the ta depends. Titration Acidity Formula.
From chemistryguru.com.sg
Titration Curve for Weak Acid Strong Base Titration Acidity Formula Acids, ta analysis is one of the most basic analyses in a winery lab. The volume of the titrant added. The acid content impacts the taste, color, and microbial stability. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. To evaluate the relationship between a titration’s equivalence point and. Titration Acidity Formula.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration Acidity Formula Acids, ta analysis is one of the most basic analyses in a winery lab. The volume of the titrant added. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The. Titration Acidity Formula.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Acidity Formula To evaluate the relationship between a titration’s equivalence point and its end point we need to. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Technically, titratable acidity is the sum of all of the free (dissociated) and bound (undissociated) protons in a solution. Calculating the ta depends on. Titration Acidity Formula.
From www.youtube.com
pH titration curve calculations for weak acid strong base YouTube Titration Acidity Formula The acid content impacts the taste, color, and microbial stability. The volume of the titrant added. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Calculating the ta depends. Titration Acidity Formula.
From collegedunia.com
Titration Formula Types, Process & Acid Base Indicators Titration Acidity Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The volume of the titrant added. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The acid content impacts the taste, color, and microbial stability. The above equation works only for. Titration Acidity Formula.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Acidity Formula Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The acid content impacts the taste, color, and microbial stability. Technically, titratable acidity is the sum of all of the free (dissociated). Titration Acidity Formula.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Calculations Chemistry Titration Acidity Formula Acids, ta analysis is one of the most basic analyses in a winery lab. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The following example exercise demonstrates the computation of ph for a titration. Titration Acidity Formula.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Acidity Formula The example below demonstrates the. The acid content impacts the taste, color, and microbial stability. To evaluate the relationship between a titration’s equivalence point and its end point we need to. The volume of the titrant added. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculating the ta. Titration Acidity Formula.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID2145680 Titration Acidity Formula Acids, ta analysis is one of the most basic analyses in a winery lab. Calculating the ta depends on the sample size you titrate and the concentration of sodium hydroxide you use. The example below demonstrates the. The acid content impacts the taste, color, and microbial stability. Technically, titratable acidity is the sum of all of the free (dissociated) and. Titration Acidity Formula.