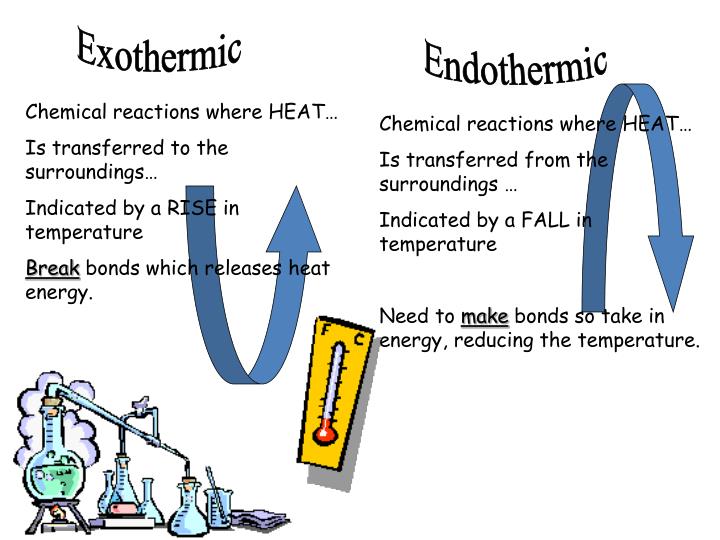
Why Is Liquid To Gas Endothermic . A liquid can freeze into a solid or vaporize into a gas. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. Endothermic conversion of liquid to gas. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. All phase changes are accompanied by changes in the energy of a system. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. All phase changes are accompanied by changes in the energy of a system. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat.
from www.slideserve.com
A gas can deposit into a solid, condense into a liquid, or ionize into plasma. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. All phase changes are accompanied by changes in the energy of a system. All phase changes are accompanied by changes in the energy of a system. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. A liquid can freeze into a solid or vaporize into a gas. Endothermic conversion of liquid to gas.
PPT Endothermic Reaction Investigation Ammonium Chloride + Water PowerPoint Presentation
Why Is Liquid To Gas Endothermic Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. All phase changes are accompanied by changes in the energy of a system. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. A liquid can freeze into a solid or vaporize into a gas. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. All phase changes are accompanied by changes in the energy of a system. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. Endothermic conversion of liquid to gas.
From www.snexplores.org
Explainer What are the different states of matter? Why Is Liquid To Gas Endothermic Endothermic conversion of liquid to gas. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. A liquid can freeze into a solid or vaporize into a gas. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. When. Why Is Liquid To Gas Endothermic.
From slideplayer.com
States of Matter. ppt download Why Is Liquid To Gas Endothermic Endothermic conversion of liquid to gas. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. All phase changes are accompanied by changes in the energy of a system. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. Vaporization requires. Why Is Liquid To Gas Endothermic.
From slideplayer.com
Water Properties Chapter ppt download Why Is Liquid To Gas Endothermic Endothermic conversion of liquid to gas. All phase changes are accompanied by changes in the energy of a system. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. All phase changes are accompanied by changes in the energy of a system. This means that as you. Why Is Liquid To Gas Endothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Why Is Liquid To Gas Endothermic Endothermic conversion of liquid to gas. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. All phase changes are accompanied by changes in the energy of a system. This means that. Why Is Liquid To Gas Endothermic.
From www.slideshare.net
Unit 1 Presentation Why Is Liquid To Gas Endothermic Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. All phase changes are accompanied by changes in the energy of a system. All phase changes are accompanied by changes in the energy of a system. A gas can deposit into a solid, condense into a liquid,. Why Is Liquid To Gas Endothermic.
From sciencenotes.org
Endothermic Reactions Definition and Examples Why Is Liquid To Gas Endothermic Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. A liquid can freeze into a solid or vaporize into a gas. Endothermic conversion of liquid to gas. All. Why Is Liquid To Gas Endothermic.
From kunduz.com
[ANSWERED] Question 7 Which phase change is endothermic gas to liquid Kunduz Why Is Liquid To Gas Endothermic This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. All phase changes are accompanied by changes in the energy of a system. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. All phase changes are accompanied by changes in. Why Is Liquid To Gas Endothermic.
From slideplayer.com
Vapor Pressure Vaporization change from liquid to gas at boiling point. Evaporation change Why Is Liquid To Gas Endothermic Endothermic conversion of liquid to gas. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. A liquid can freeze into a solid or vaporize into a gas. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. All phase changes are accompanied by changes in the. Why Is Liquid To Gas Endothermic.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Why Is Liquid To Gas Endothermic A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Endothermic conversion of liquid to gas. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. A liquid can freeze into a solid or vaporize into a gas. All phase changes are accompanied by. Why Is Liquid To Gas Endothermic.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3527416 Why Is Liquid To Gas Endothermic All phase changes are accompanied by changes in the energy of a system. Endothermic conversion of liquid to gas. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. A liquid can freeze into. Why Is Liquid To Gas Endothermic.
From www.thoughtco.com
Endothermic Reaction Examples Why Is Liquid To Gas Endothermic When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. Endothermic conversion of liquid to gas. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to. Why Is Liquid To Gas Endothermic.
From www.slideserve.com
PPT Chapter 6 Notes PowerPoint Presentation, free download ID5812761 Why Is Liquid To Gas Endothermic A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the. Why Is Liquid To Gas Endothermic.
From slideplayer.com
Liquids, Solids and Changes of State ppt video online download Why Is Liquid To Gas Endothermic Endothermic conversion of liquid to gas. All phase changes are accompanied by changes in the energy of a system. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular.. Why Is Liquid To Gas Endothermic.
From higheducationlearning.com
Is Sublimation Endothermic Or Exothermic Process? » Education Tips Why Is Liquid To Gas Endothermic A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have. Why Is Liquid To Gas Endothermic.
From slideplayer.com
Enthalpy and Chemical Reactions ppt download Why Is Liquid To Gas Endothermic This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. All phase changes are accompanied by changes in the energy of a system. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. All phase changes. Why Is Liquid To Gas Endothermic.
From www.numerade.com
SOLVED Please help!! Draw the phase diagram for water. Point to the region where water changes Why Is Liquid To Gas Endothermic Endothermic conversion of liquid to gas. All phase changes are accompanied by changes in the energy of a system. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular.. Why Is Liquid To Gas Endothermic.
From slideplayer.com
Water Properties Chapter ppt download Why Is Liquid To Gas Endothermic A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. All phase changes are accompanied by changes in the energy of a system. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. This means that as you move. Why Is Liquid To Gas Endothermic.
From improove.in
Experiment 16 Heat generation during reaction Improove Why Is Liquid To Gas Endothermic All phase changes are accompanied by changes in the energy of a system. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. All phase changes are accompanied by changes in the energy of a system. This means that as you move from solid to liquid to gas, all accompanying phase changes. Why Is Liquid To Gas Endothermic.
From pamelakruwhiggins.blogspot.com
Which of the Following Phase Changes Is an Endothermic Change PamelakruwHiggins Why Is Liquid To Gas Endothermic All phase changes are accompanied by changes in the energy of a system. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. All phase changes are accompanied by changes in the energy of a system. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from. Why Is Liquid To Gas Endothermic.
From slideplayer.com
States of Matter and Phase Changes ppt download Why Is Liquid To Gas Endothermic A liquid can freeze into a solid or vaporize into a gas. All phase changes are accompanied by changes in the energy of a system. Endothermic conversion of liquid to gas. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. Vaporization requires energy to break the. Why Is Liquid To Gas Endothermic.
From maxim-kwise.blogspot.com
The Process by Which a Gas Changes Into a Liquid Why Is Liquid To Gas Endothermic This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. All phase changes are accompanied by. Why Is Liquid To Gas Endothermic.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID6364049 Why Is Liquid To Gas Endothermic When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. A liquid can freeze into a solid or vaporize into a gas. All phase changes are accompanied by changes in the energy of a system. Endothermic conversion of liquid to gas. Vaporization requires energy to break the. Why Is Liquid To Gas Endothermic.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Why Is Liquid To Gas Endothermic This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. Endothermic conversion of liquid to gas. A gas can deposit into a solid, condense into a liquid, or ionize into plasma. A liquid can freeze into a solid or vaporize into a gas. All phase changes are accompanied by. Why Is Liquid To Gas Endothermic.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Why Is Liquid To Gas Endothermic When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. All phase changes are accompanied by changes in the energy of a system. Endothermic conversion of liquid to gas. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as. Why Is Liquid To Gas Endothermic.
From slideplayer.com
MATTER STATES OF. ppt download Why Is Liquid To Gas Endothermic A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. All phase changes are accompanied by changes in the energy of a system. A gas can deposit into a solid, condense. Why Is Liquid To Gas Endothermic.
From www.slideserve.com
PPT Endothermic vs. Exothermic Reactions PowerPoint Presentation ID2455578 Why Is Liquid To Gas Endothermic A gas can deposit into a solid, condense into a liquid, or ionize into plasma. Endothermic conversion of liquid to gas. All phase changes are accompanied by changes in the energy of a system. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. All phase changes are accompanied by changes in. Why Is Liquid To Gas Endothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Why Is Liquid To Gas Endothermic When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. All phase changes are accompanied by changes in the energy of a system. A. Why Is Liquid To Gas Endothermic.
From www.slideshare.net
States of matter Why Is Liquid To Gas Endothermic When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. All phase changes are accompanied by changes in the energy of a system. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. A. Why Is Liquid To Gas Endothermic.
From slideplayer.com
Phases of Matter, Energy and Phase Changes ppt download Why Is Liquid To Gas Endothermic A liquid can freeze into a solid or vaporize into a gas. Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. All phase changes are accompanied by changes in the energy of a system. When a liquid (in my case i used butane) under high pressure. Why Is Liquid To Gas Endothermic.
From www.gbu-presnenskij.ru
For The Three States Of Matter (solid, Liquid, And Gas), 50 OFF Why Is Liquid To Gas Endothermic All phase changes are accompanied by changes in the energy of a system. Endothermic conversion of liquid to gas. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. A liquid can freeze into a solid or vaporize into a gas. A gas can deposit into a solid, condense. Why Is Liquid To Gas Endothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Why Is Liquid To Gas Endothermic Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. All phase changes are accompanied by changes in the energy of a system. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. All phase changes are accompanied by. Why Is Liquid To Gas Endothermic.
From www.slideserve.com
PPT Endothermic Reaction Investigation Ammonium Chloride + Water PowerPoint Presentation Why Is Liquid To Gas Endothermic This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. A liquid can freeze into a solid or vaporize into a gas. A gas can deposit into a solid, condense into. Why Is Liquid To Gas Endothermic.
From www.reddit.com
Logic of Exothermic, Endothermic and Entropy So, Going from a gas to a liquid and liquid to a Why Is Liquid To Gas Endothermic A liquid can freeze into a solid or vaporize into a gas. When a liquid (in my case i used butane) under high pressure is released into the air, it becomes gaseous, and the container. A liquid gas phase change (vaporization) is endothermic because energy is absorbed from the surroundings to overcome intermolecular. A gas can deposit into a solid,. Why Is Liquid To Gas Endothermic.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation, free download ID5913704 Why Is Liquid To Gas Endothermic Vaporization requires energy to break the bonds holding a molecule together in a liquid, condensation releases energy as liquid molecules have much less. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. All phase changes are accompanied by changes in the energy of a system. A liquid gas. Why Is Liquid To Gas Endothermic.
From www.britannica.com
phase Definition & Facts Britannica Why Is Liquid To Gas Endothermic All phase changes are accompanied by changes in the energy of a system. All phase changes are accompanied by changes in the energy of a system. This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. A liquid can freeze into a solid or vaporize into a gas. Vaporization. Why Is Liquid To Gas Endothermic.