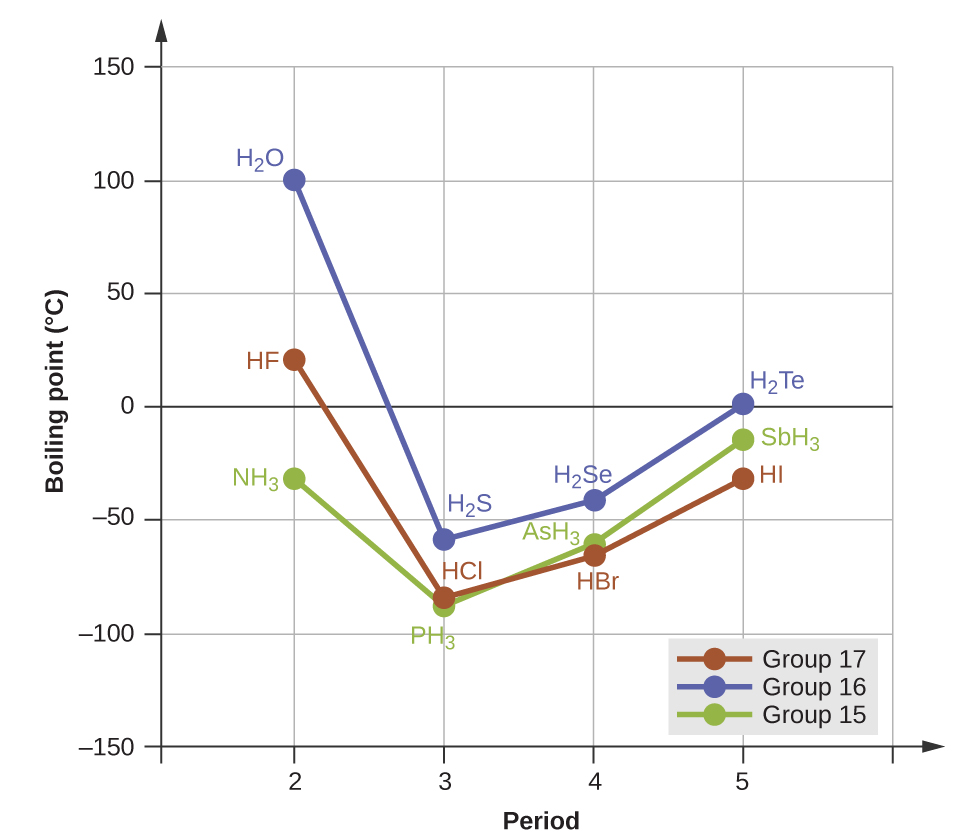
Lower Boiling Point Than Bromine . If you explore the graphs, you will find that fluorine and chlorine are. because boiling point of different materials depend on the intermolecular forces present between the atoms. The stronger the imfs, the lower the vapor pressure of the substance. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. However, as molecular weight increases, boiling point also goes up. The stronger the intermolecular forces the. you will see that both melting points and boiling points rise as you go down the group. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. — the halogens have low melting points and low boiling points. — intermolecular forces (imfs) can be used to predict relative boiling points.
from wisc.pb.unizin.org
The stronger the imfs, the lower the vapor pressure of the substance. The stronger the intermolecular forces the. If you explore the graphs, you will find that fluorine and chlorine are. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. However, as molecular weight increases, boiling point also goes up. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. — the halogens have low melting points and low boiling points. you will see that both melting points and boiling points rise as you go down the group. because boiling point of different materials depend on the intermolecular forces present between the atoms. — intermolecular forces (imfs) can be used to predict relative boiling points.
M10Q2 Melting and Boiling Point Comparisons Chem 103/104 Resource Book
Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. — the halogens have low melting points and low boiling points. If you explore the graphs, you will find that fluorine and chlorine are. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. However, as molecular weight increases, boiling point also goes up. The stronger the imfs, the lower the vapor pressure of the substance. The stronger the intermolecular forces the. you will see that both melting points and boiling points rise as you go down the group. — intermolecular forces (imfs) can be used to predict relative boiling points. because boiling point of different materials depend on the intermolecular forces present between the atoms.
From www.gauthmath.com
Solved The boiling points of diatomic halogens are compared in the Lower Boiling Point Than Bromine If you explore the graphs, you will find that fluorine and chlorine are. you will see that both melting points and boiling points rise as you go down the group. — intermolecular forces (imfs) can be used to predict relative boiling points. because boiling point of different materials depend on the intermolecular forces present between the atoms.. Lower Boiling Point Than Bromine.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Lower Boiling Point Than Bromine The stronger the imfs, the lower the vapor pressure of the substance. — the halogens have low melting points and low boiling points. If you explore the graphs, you will find that fluorine and chlorine are. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room. Lower Boiling Point Than Bromine.
From sitionroll.blogspot.com
How To Determine Boiling Point Of A Compound sition Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. — intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the intermolecular forces the. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. . Lower Boiling Point Than Bromine.
From www.youtube.com
4 2 6 aldehydes and ketones boiling point and solubility YouTube Lower Boiling Point Than Bromine If you explore the graphs, you will find that fluorine and chlorine are. The stronger the intermolecular forces the. you will see that both melting points and boiling points rise as you go down the group. The stronger the imfs, the lower the vapor pressure of the substance. because boiling point of different materials depend on the intermolecular. Lower Boiling Point Than Bromine.
From www.youtube.com
Boiling Point of NH3 and H2O (Explanation of Difference) YouTube Lower Boiling Point Than Bromine A small molecule like methane has very weak intermolecular forces, and has a low boiling point. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. you will see that both melting points and boiling points rise as you go down the group. —. Lower Boiling Point Than Bromine.
From www.slideserve.com
PPT Properties of Aldehydes and Ketones PowerPoint Presentation, free Lower Boiling Point Than Bromine If you explore the graphs, you will find that fluorine and chlorine are. because boiling point of different materials depend on the intermolecular forces present between the atoms. — the halogens have low melting points and low boiling points. However, as molecular weight increases, boiling point also goes up. — intermolecular forces (imfs) can be used to. Lower Boiling Point Than Bromine.
From manualfixnodfounderous.z13.web.core.windows.net
Chemistry Boiling Point Chart Lower Boiling Point Than Bromine The stronger the intermolecular forces the. If you explore the graphs, you will find that fluorine and chlorine are. However, as molecular weight increases, boiling point also goes up. — intermolecular forces (imfs) can be used to predict relative boiling points. you will see that both melting points and boiling points rise as you go down the group.. Lower Boiling Point Than Bromine.
From www.numerade.com
SOLVED (6). Define distillation? boiling liquids condensing liquids Lower Boiling Point Than Bromine However, as molecular weight increases, boiling point also goes up. — the halogens have low melting points and low boiling points. The stronger the imfs, the lower the vapor pressure of the substance. — intermolecular forces (imfs) can be used to predict relative boiling points. you will see that both melting points and boiling points rise as. Lower Boiling Point Than Bromine.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Lower Boiling Point Than Bromine However, as molecular weight increases, boiling point also goes up. — intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the intermolecular forces the. — the halogens have low melting points and low boiling points. The stronger the imfs, the lower the vapor pressure of the substance. If you explore the graphs, you will. Lower Boiling Point Than Bromine.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Lower Boiling Point Than Bromine — intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the intermolecular forces the. The stronger the imfs, the lower the vapor pressure of the substance. If you explore the graphs, you will find that fluorine and chlorine are. However, as molecular weight increases, boiling point also goes up. because boiling point of different. Lower Boiling Point Than Bromine.
From www.numerade.com
SOLVED(a) Use data in Appendix C to estimate the boiling point of Lower Boiling Point Than Bromine — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. — the halogens have low melting points and low boiling points. you will see that both melting points and boiling points rise as you go down the group. If you explore the graphs, you. Lower Boiling Point Than Bromine.
From wou.edu
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. The stronger the intermolecular forces the. The stronger the imfs, the lower the vapor pressure of the substance. If you explore the graphs, you will find that fluorine. Lower Boiling Point Than Bromine.
From infogram.com
Top 10 Elements with the lowest boiling point Infogram Lower Boiling Point Than Bromine However, as molecular weight increases, boiling point also goes up. — the halogens have low melting points and low boiling points. because boiling point of different materials depend on the intermolecular forces present between the atoms. The stronger the intermolecular forces the. A small molecule like methane has very weak intermolecular forces, and has a low boiling point.. Lower Boiling Point Than Bromine.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Lower Boiling Point Than Bromine — intermolecular forces (imfs) can be used to predict relative boiling points. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. because boiling point of different materials depend on the intermolecular forces present between the atoms. The stronger the intermolecular forces the. — the halogens all have low melting and. Lower Boiling Point Than Bromine.
From www.nuclear-power.com
Bromine Melting Point Boiling Point Lower Boiling Point Than Bromine However, as molecular weight increases, boiling point also goes up. If you explore the graphs, you will find that fluorine and chlorine are. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. you will see that both melting points and boiling points rise as. Lower Boiling Point Than Bromine.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. However, as molecular weight increases, boiling point also goes up. If you explore the graphs, you will find that fluorine and chlorine are. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids. Lower Boiling Point Than Bromine.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols Lower Boiling Point Than Bromine However, as molecular weight increases, boiling point also goes up. If you explore the graphs, you will find that fluorine and chlorine are. — the halogens have low melting points and low boiling points. The stronger the imfs, the lower the vapor pressure of the substance. — intermolecular forces (imfs) can be used to predict relative boiling points.. Lower Boiling Point Than Bromine.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Lower Boiling Point Than Bromine — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. The stronger the imfs, the lower the vapor pressure of the substance. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. If you explore the graphs, you will find. Lower Boiling Point Than Bromine.
From www.coursehero.com
[Solved] The boiling point of bromine is 59 "C. Which of the following Lower Boiling Point Than Bromine A small molecule like methane has very weak intermolecular forces, and has a low boiling point. If you explore the graphs, you will find that fluorine and chlorine are. you will see that both melting points and boiling points rise as you go down the group. — the halogens all have low melting and boiling points which means. Lower Boiling Point Than Bromine.
From www.slideserve.com
PPT Chapter 2; Hydrocarbon Frameworks; Alkanes PowerPoint Lower Boiling Point Than Bromine you will see that both melting points and boiling points rise as you go down the group. The stronger the intermolecular forces the. However, as molecular weight increases, boiling point also goes up. The stronger the imfs, the lower the vapor pressure of the substance. — intermolecular forces (imfs) can be used to predict relative boiling points. A. Lower Boiling Point Than Bromine.
From www.gauthmath.com
Solved The boiling points of diatomic halogens are compared in the Lower Boiling Point Than Bromine A small molecule like methane has very weak intermolecular forces, and has a low boiling point. If you explore the graphs, you will find that fluorine and chlorine are. because boiling point of different materials depend on the intermolecular forces present between the atoms. The stronger the imfs, the lower the vapor pressure of the substance. The stronger the. Lower Boiling Point Than Bromine.
From www.numerade.com
SOLVEDFrom data in Appendix J, estimate (a) the boiling point of Lower Boiling Point Than Bromine The stronger the imfs, the lower the vapor pressure of the substance. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. you will see that both melting points and boiling points rise as you go down the group. The stronger the intermolecular forces the. — the halogens have low melting points. Lower Boiling Point Than Bromine.
From www.numerade.com
SOLVED Calculate the boiling point of bromine, Br2 from the data given Lower Boiling Point Than Bromine — intermolecular forces (imfs) can be used to predict relative boiling points. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. — the halogens have low melting. Lower Boiling Point Than Bromine.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts Lower Boiling Point Than Bromine — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. because boiling point of different materials depend on the intermolecular forces present between the atoms. However, as molecular weight. Lower Boiling Point Than Bromine.
From ar.inspiredpencil.com
Intermolecular Forces Hf Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. — intermolecular forces (imfs) can be used to predict relative boiling points. A small molecule like methane has very. Lower Boiling Point Than Bromine.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Lower Boiling Point Than Bromine The stronger the intermolecular forces the. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. If you explore the graphs, you will find that fluorine and chlorine are. because boiling point of different materials depend on the intermolecular forces present between the atoms. However,. Lower Boiling Point Than Bromine.
From dxojgfcke.blob.core.windows.net
What Is The Boiling Point And Freezing Point Of Water On Kelvin Scale Lower Boiling Point Than Bromine The stronger the intermolecular forces the. However, as molecular weight increases, boiling point also goes up. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. The stronger the imfs, the lower the vapor pressure of the substance. — intermolecular forces (imfs) can be used to predict relative boiling points. — the. Lower Boiling Point Than Bromine.
From chemguru.sg
2020 P1 Q4 Compare Boiling Points of C5H12 Isomers Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. However, as molecular weight increases, boiling point also goes up. — the halogens all have low melting and boiling points which means that they tend to be. Lower Boiling Point Than Bromine.
From www.chegg.com
Solved The normal boiling point (at P=1 bar) of bromine Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. The stronger the intermolecular forces the. The stronger the imfs, the lower the vapor pressure of the substance. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. —. Lower Boiling Point Than Bromine.
From www.numerade.com
SOLVED The picture below represents the particle of a compound. The Lower Boiling Point Than Bromine because boiling point of different materials depend on the intermolecular forces present between the atoms. — the halogens have low melting points and low boiling points. — intermolecular forces (imfs) can be used to predict relative boiling points. If you explore the graphs, you will find that fluorine and chlorine are. — the halogens all have. Lower Boiling Point Than Bromine.
From www.numerade.com
SOLVED Use molecular structure and intermolecular bonding to describe Lower Boiling Point Than Bromine However, as molecular weight increases, boiling point also goes up. The stronger the imfs, the lower the vapor pressure of the substance. you will see that both melting points and boiling points rise as you go down the group. — the halogens have low melting points and low boiling points. If you explore the graphs, you will find. Lower Boiling Point Than Bromine.
From wisc.pb.unizin.org
M10Q2 Melting and Boiling Point Comparisons Chem 103/104 Resource Book Lower Boiling Point Than Bromine However, as molecular weight increases, boiling point also goes up. The stronger the intermolecular forces the. — intermolecular forces (imfs) can be used to predict relative boiling points. A small molecule like methane has very weak intermolecular forces, and has a low boiling point. — the halogens all have low melting and boiling points which means that they. Lower Boiling Point Than Bromine.
From material-properties.org
Bromine Thermal Properties Melting Point Thermal Conductivity Lower Boiling Point Than Bromine you will see that both melting points and boiling points rise as you go down the group. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. — intermolecular forces (imfs) can be used to predict relative boiling points. — the halogens have. Lower Boiling Point Than Bromine.
From www.youtube.com
Melting and Boiling points, Chemistry Lecture Sabaq.pk YouTube Lower Boiling Point Than Bromine A small molecule like methane has very weak intermolecular forces, and has a low boiling point. — the halogens have low melting points and low boiling points. — intermolecular forces (imfs) can be used to predict relative boiling points. because boiling point of different materials depend on the intermolecular forces present between the atoms. — the. Lower Boiling Point Than Bromine.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Lower Boiling Point Than Bromine The stronger the imfs, the lower the vapor pressure of the substance. — the halogens all have low melting and boiling points which means that they tend to be gases or liquids at room temperature. you will see that both melting points and boiling points rise as you go down the group. because boiling point of different. Lower Boiling Point Than Bromine.