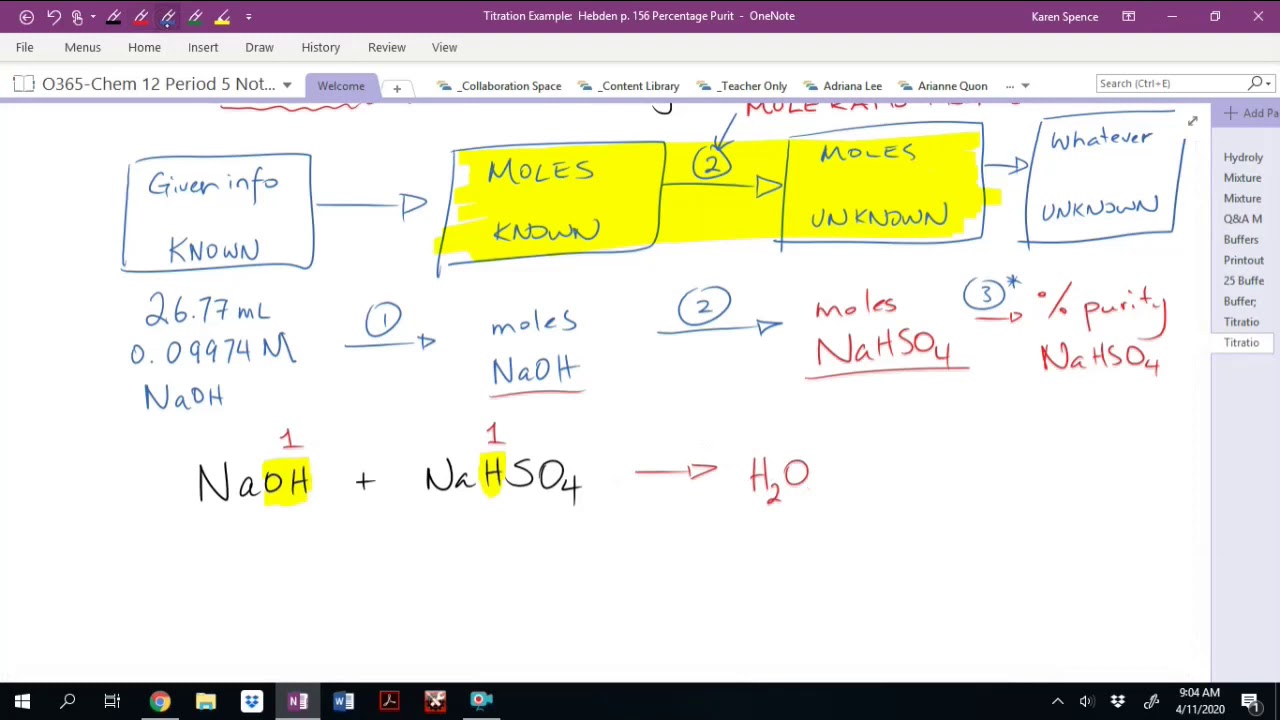
Titration Calculations Percentage Purity . determination of percentage purity. Titration between nitric acid and sodium carbonate is represented by the equation. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. Writing a balanced chemical equation. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. When an impure chemical substance is used in titration, only the pure part of that. % purity is the percentage of the material which is the actually desired chemical in a sample of it.
from www.youtube.com
Titration between nitric acid and sodium carbonate is represented by the equation. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. % purity is the percentage of the material which is the actually desired chemical in a sample of it. Writing a balanced chemical equation. When an impure chemical substance is used in titration, only the pure part of that. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. determination of percentage purity.
Titration Calculations & Percent Purity YouTube
Titration Calculations Percentage Purity n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. Writing a balanced chemical equation. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. % purity is the percentage of the material which is the actually desired chemical in a sample of it. Titration between nitric acid and sodium carbonate is represented by the equation. determination of percentage purity. When an impure chemical substance is used in titration, only the pure part of that. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of.
From www.youtube.com
Percent Composition and Purity Titration Calculations Solved Titration Calculations Percentage Purity % purity is the percentage of the material which is the actually desired chemical in a sample of it. Titration between nitric acid and sodium carbonate is represented by the equation. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When an impure chemical substance is used in titration, only. Titration Calculations Percentage Purity.
From www.youtube.com
percent purity titration YouTube Titration Calculations Percentage Purity determination of percentage purity. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. When an impure chemical. Titration Calculations Percentage Purity.
From www.nagwa.com
Question Video Determining the Equation to Calculate Percentage Purity Titration Calculations Percentage Purity determination of percentage purity. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. n (mol) = c (mol /l) * v (l) and the amount of titrant can. Titration Calculations Percentage Purity.
From exosjwdrb.blob.core.windows.net
Titration Calculation Table Method at Susan Clyburn blog Titration Calculations Percentage Purity At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Writing a balanced chemical equation. When an impure chemical substance is used in titration, only the pure part of that. % purity is the percentage of the material which is the actually desired chemical in a sample of it. Titration between. Titration Calculations Percentage Purity.
From www.youtube.com
REDOX TITRATION CALCULATION MOLARITY AND PERCENTAGE PURITY KMNO4VS Titration Calculations Percentage Purity Titration between nitric acid and sodium carbonate is represented by the equation. determination of percentage purity. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When an impure chemical substance is used in titration, only the pure part of that. Writing a balanced chemical equation. % purity is the. Titration Calculations Percentage Purity.
From www.coursehero.com
[Solved] how should I calculate the percentage purity of aspirin Titration Calculations Percentage Purity Titration between nitric acid and sodium carbonate is represented by the equation. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. determination of percentage purity. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. Titration Calculations Percentage Purity.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration Calculations Percentage Purity % purity is the percentage of the material which is the actually desired chemical in a sample of it. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Titration Calculations Percentage Purity.
From exohtntjr.blob.core.windows.net
How To Calculate Titration Value at Eric Whitlow blog Titration Calculations Percentage Purity n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. When an impure chemical substance is used in titration, only the pure part of that. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration Calculations Percentage Purity.
From dxowlwwmg.blob.core.windows.net
Titration Experiment Titration Results Table at Barbara Dorman blog Titration Calculations Percentage Purity Writing a balanced chemical equation. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. for example if we are asked to. Titration Calculations Percentage Purity.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Calculations Percentage Purity for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. determination of percentage purity. Titration between nitric acid. Titration Calculations Percentage Purity.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration Calculations Percentage Purity When an impure chemical substance is used in titration, only the pure part of that. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount. Titration Calculations Percentage Purity.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Calculations Percentage Purity volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. Titration between nitric acid and sodium carbonate is represented by the equation. At the equivalence point in a neutralization, the moles. Titration Calculations Percentage Purity.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Calculations Percentage Purity At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. determination of percentage purity. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction. Titration Calculations Percentage Purity.
From www.youtube.com
Back Titration and Percentage Purity YouTube Titration Calculations Percentage Purity for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. Writing a balanced chemical equation. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration Calculations Percentage Purity.
From www.youtube.com
Grade 12 Acids&Bases Percentage Purity Titration Calculation IEB Titration Calculations Percentage Purity At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. determination of percentage purity. volumetric analysis is a chemical analytical procedure. Titration Calculations Percentage Purity.
From www.youtube.com
How to solve for the Purity of substances after titration YouTube Titration Calculations Percentage Purity Writing a balanced chemical equation. When an impure chemical substance is used in titration, only the pure part of that. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration between nitric acid and sodium carbonate is represented by the equation. for example if we are asked to find a. Titration Calculations Percentage Purity.
From www.youtube.com
Titration Calculations & Percent Purity YouTube Titration Calculations Percentage Purity At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Writing a balanced chemical equation. determination of percentage purity. Titration between nitric acid and sodium carbonate is represented by the equation. for example if we are asked to find a purity of the substance, we must convert concentration found to. Titration Calculations Percentage Purity.
From www.youtube.com
Percentage purity from manganate (VII) titrations (using DMEMA Titration Calculations Percentage Purity Titration between nitric acid and sodium carbonate is represented by the equation. determination of percentage purity. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. for example if we are asked to. Titration Calculations Percentage Purity.
From www.youtube.com
Percent purity lesson YouTube Titration Calculations Percentage Purity volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte. When an impure chemical substance is used in titration, only the pure part of. Titration Calculations Percentage Purity.
From keplarllp.com
️ How to calculate percentage purity of an impure sample. How to Titration Calculations Percentage Purity for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. % purity is the percentage of the material which is the actually desired chemical in a sample of it. Writing a balanced chemical equation. determination of percentage purity. At the equivalence point in a neutralization, the. Titration Calculations Percentage Purity.
From www.youtube.com
Determination of Percentage Composition/Purity of a MixtureVolumetric Titration Calculations Percentage Purity When an impure chemical substance is used in titration, only the pure part of that. % purity is the percentage of the material which is the actually desired chemical in a sample of it. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation to determine. Titration Calculations Percentage Purity.
From www.youtube.com
Percentage purity from titrations YouTube Titration Calculations Percentage Purity At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. determination of percentage purity. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. for example if we are asked to find a purity of the substance, we must convert concentration found to. Titration Calculations Percentage Purity.
From keplarllp.com
️ How to calculate percentage purity of an impure sample. How to Titration Calculations Percentage Purity for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. Writing a balanced chemical equation. Titration between nitric acid and sodium carbonate is represented by the equation. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. determination of percentage. Titration Calculations Percentage Purity.
From www.slideserve.com
PPT Unit 7 PowerPoint Presentation, free download ID5948396 Titration Calculations Percentage Purity When an impure chemical substance is used in titration, only the pure part of that. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. % purity is the percentage of the material which is the actually desired chemical in a sample of it. At the equivalence. Titration Calculations Percentage Purity.
From exosqdnkw.blob.core.windows.net
Titration Calculations A Level Aqa at John Walling blog Titration Calculations Percentage Purity for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. When an impure chemical substance is used in titration, only the pure part of that. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric calculation. Titration Calculations Percentage Purity.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculations Percentage Purity When an impure chemical substance is used in titration, only the pure part of that. Titration between nitric acid and sodium carbonate is represented by the equation. determination of percentage purity. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. n (mol) = c (mol /l) * v (l) and. Titration Calculations Percentage Purity.
From www.youtube.com
As Chemistry How to calculate the percentage Purity by Titration (part Titration Calculations Percentage Purity % purity is the percentage of the material which is the actually desired chemical in a sample of it. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. When. Titration Calculations Percentage Purity.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Calculations Percentage Purity When an impure chemical substance is used in titration, only the pure part of that. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. Writing a balanced chemical equation. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration between nitric acid and. Titration Calculations Percentage Purity.
From www.youtube.com
How to Calculate Percentage Purity Step by Step Tutorial (IGCSE/OLevel Titration Calculations Percentage Purity Titration between nitric acid and sodium carbonate is represented by the equation. determination of percentage purity. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. Writing a balanced chemical. Titration Calculations Percentage Purity.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculations Percentage Purity determination of percentage purity. Writing a balanced chemical equation. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration between nitric acid and sodium carbonate is represented by the equation. n (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. Titration Calculations Percentage Purity.
From www.studocu.com
Experiment 3 Redox Titration Percent Purity Analysis Experiment Three Titration Calculations Percentage Purity When an impure chemical substance is used in titration, only the pure part of that. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. Titration between nitric acid and. Titration Calculations Percentage Purity.
From www.numerade.com
SOLVED Calculate the percent purity of a sample of Mg(OH)2 if Titration Calculations Percentage Purity volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. When an impure chemical substance is used in titration, only the pure part of that. Writing a balanced chemical equation. . Titration Calculations Percentage Purity.
From dxojrsulq.blob.core.windows.net
How To Report A Titration Experiment at Joseph Heitmann blog Titration Calculations Percentage Purity Titration between nitric acid and sodium carbonate is represented by the equation. determination of percentage purity. When an impure chemical substance is used in titration, only the pure part of that. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. for example if we are asked to find a purity. Titration Calculations Percentage Purity.
From www.youtube.com
Titration calculation Formula for percentage purity or Standardization Titration Calculations Percentage Purity % purity is the percentage of the material which is the actually desired chemical in a sample of it. for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. When an impure chemical substance is used in titration, only the pure part of that. n (mol). Titration Calculations Percentage Purity.
From www.chegg.com
Solved Table 2 Titration Data for the Percentage Purity of Titration Calculations Percentage Purity for example if we are asked to find a purity of the substance, we must convert concentration found to amount of. determination of percentage purity. volumetric analysis is a chemical analytical procedure based on measurement of volumes of reaction in solutions. When an impure chemical substance is used in titration, only the pure part of that. At. Titration Calculations Percentage Purity.