Microbial Test Ep . it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the following chapter is published for information. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. This general chapter presents 2 sets of recommended acceptance criteria for. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for.
from www.qimedical.com
This general chapter presents 2 sets of recommended acceptance criteria for. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the following chapter is published for information. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities.
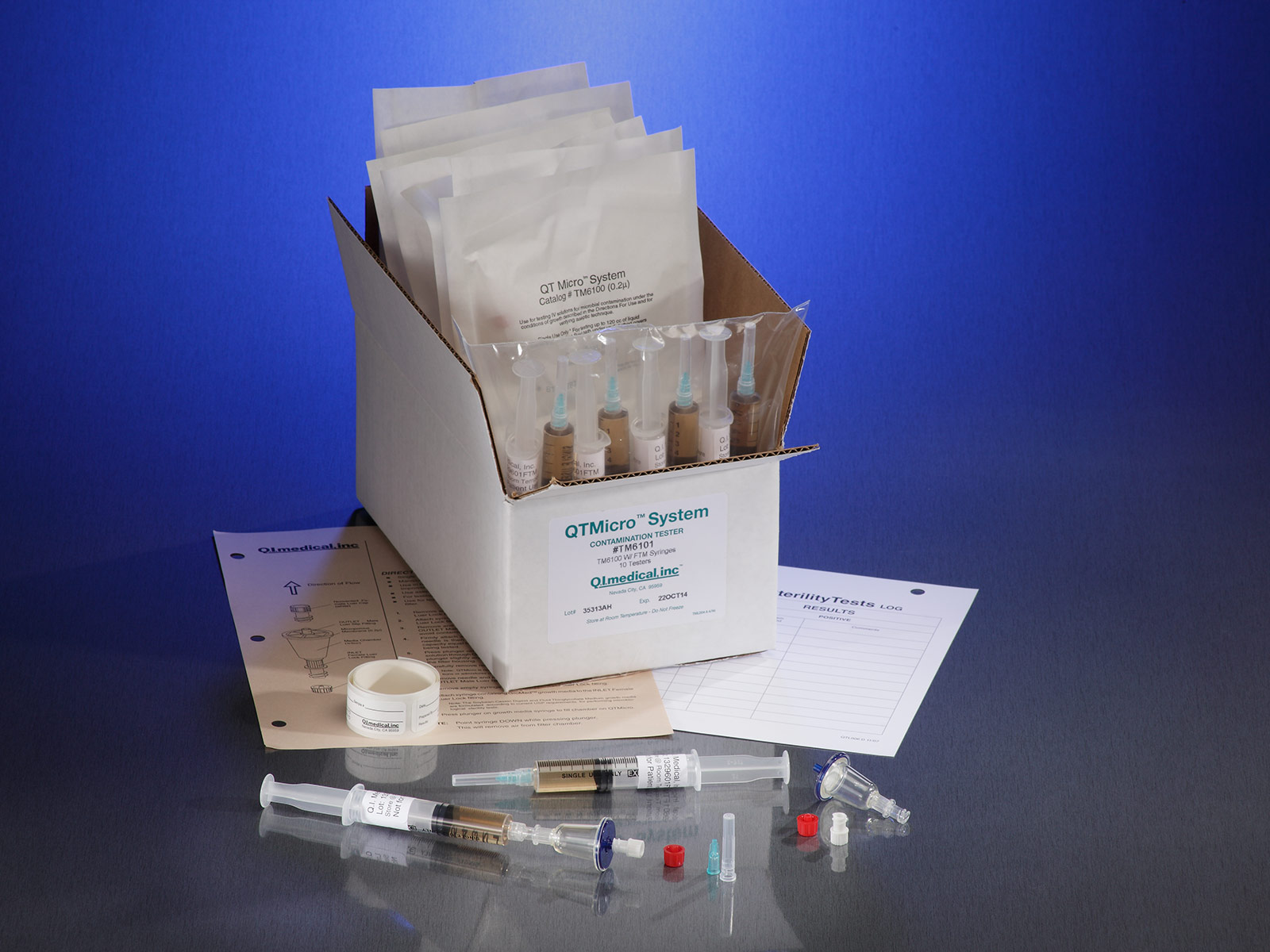
QT Micro™ Microbial Contamination Test Kit QI Medical, Inc.
Microbial Test Ep it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the following chapter is published for information. This general chapter presents 2 sets of recommended acceptance criteria for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities.
From candl.org
HYLiTE® Rapid Microbial Test ECHA Microbiology Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. This general chapter presents 2 sets of recommended acceptance criteria for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the tests are designed primarily to determine whether a substance or preparation complies. Microbial Test Ep.
From microbiologyguideritesh.com
Fundamentals of Microbial Limit Test 2023 Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the following chapter is published. Microbial Test Ep.
From www.slideshare.net
Amatsigroup Infographics Microbial Testing for Quality Control Microbial Test Ep This general chapter presents 2 sets of recommended acceptance criteria for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi. Microbial Test Ep.
From www.youtube.com
Difference Between BIOBURDEN TEST AND MICROBIAL LIMIT TEST YouTube Microbial Test Ep This general chapter presents 2 sets of recommended acceptance criteria for. the following chapter is published for information. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. Introduction the tests described hereafter. Microbial Test Ep.
From www.youtube.com
Do you know the procedure of Microbial Limit Test? Microbial Analysis Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. This general chapter presents 2 sets of recommended acceptance criteria for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration. Microbial Test Ep.
From www.youtube.com
Motility test Microbiology (Microbial Biochemical test) YouTube Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the following chapter is published for information. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established. Microbial Test Ep.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Microbial Test Ep it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the following chapter is published for information. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. This general chapter presents 2 sets of recommended acceptance criteria for. microbial enumeration tests are. Microbial Test Ep.
From candl.org
HYLiTE® Rapid Microbial Test ECHA Microbiology Microbial Test Ep the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. microbial enumeration tests are quantitative methods. Microbial Test Ep.
From www.slideserve.com
PPT Practical aspects of Microbiological Testing and handling of OOS Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the following chapter is published for information. the tests are designed primarily to determine whether a substance or preparation complies with an established. Microbial Test Ep.
From www.qimedical.com
QT Micro™ Microbial Contamination Test Kit QI Medical, Inc. Microbial Test Ep the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. the following chapter is published for information. This general chapter presents 2 sets of recommended acceptance criteria for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. microbial enumeration tests are. Microbial Test Ep.
From www.formulatorsampleshop.com
Microbial Test Kit, 4 Pack EX Microbial Test Ep the following chapter is published for information. This general chapter presents 2 sets of recommended acceptance criteria for. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. Introduction the tests described. Microbial Test Ep.
From cptclabs.com
Reliable Microbial Limits Test CPT Labs Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. microbial enumeration tests are quantitative methods. Microbial Test Ep.
From www.foodprocessing.com
The ABCs of Microbial Testing Food Processing Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. This general chapter presents 2 sets of recommended. Microbial Test Ep.
From www.adamsonlab.com
Microbial Testing Adamson Analytical Laboratories Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. This general chapter presents 2 sets. Microbial Test Ep.
From www.learngxp.com
Microbial Enumeration Test (MET) Explained LearnGxP Accredited Microbial Test Ep This general chapter presents 2 sets of recommended acceptance criteria for. the following chapter is published for information. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. it aims to facilitate the. Microbial Test Ep.
From etrlabs.com
The Importance of Microbial Testing for Your Industry ETR Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the tests are designed primarily to determine. Microbial Test Ep.
From medicinalgenomics.com
Cannabis Microbial Testing with qPCR A Fast, Accurate, and Scalable Microbial Test Ep the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. This general chapter presents 2 sets of recommended acceptance criteria for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration. Microbial Test Ep.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Microbial Test Ep the following chapter is published for information. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established. Microbial Test Ep.
From lotioncrafter.com
Microbial Test Kit, 4 Pack Lotioncrafter Microbial Test Ep This general chapter presents 2 sets of recommended acceptance criteria for. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the following chapter is published for information. it aims to facilitate the. Microbial Test Ep.
From www.laboratuar.com
EP 2.6.12 NonSterile Microbiological Examination, Standard Test for Microbial Test Ep the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. This general chapter presents 2 sets of. Microbial Test Ep.
From www.researchgate.net
The schematic illustration of microbial testing, including sample Microbial Test Ep the following chapter is published for information. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the tests are designed primarily to determine whether a substance or preparation complies with an established. Microbial Test Ep.
From www.diag.vn
What Is The Purpose Of Microbiological Testing? What Types Of Tests Are Microbial Test Ep it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the following chapter is published for information. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic. Microbial Test Ep.
From promptpraxislabs.com
Microbiological Testing Capabilities Analytical Testing Labs Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. This general chapter presents 2 sets of. Microbial Test Ep.
From www.researchgate.net
Identification of microbial OTUbased Ep markers by random forest Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. This general chapter presents 2 sets of recommended acceptance criteria for. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. the following chapter is published for information. microbial enumeration tests are. Microbial Test Ep.
From medicinecreekanalytics.com
Microbial Testing Medicine Creek Analytics Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. This general chapter presents 2 sets of recommended acceptance criteria for. the following chapter is published for information. it aims to facilitate the. Microbial Test Ep.
From njlabs.com
Microbiological Testing Lab NJ Labs Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. the following chapter is published for information. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory. Microbial Test Ep.
From allometrics.com
Microbial Testing Allometrics Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the following chapter is published. Microbial Test Ep.
From fixmold.com
Microbial Testing Lab FixMold Microbial Test Ep the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. This general chapter presents 2 sets. Microbial Test Ep.
From www.foodonline.com
New Microbial Testing Technology For Cleanrooms Announced By Mocon Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. Introduction the tests described hereafter will. Microbial Test Ep.
From manoxblog.com
Bioburden / Microbial Enumeration Test M A N O X B L O G Microbial Test Ep microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the following chapter is published for information.. Microbial Test Ep.
From dxosissyn.blob.core.windows.net
Microbial Test Kit 10 Pack at Susan Hess blog Microbial Test Ep it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the following chapter is published for information.. Microbial Test Ep.
From www.biosafe.fi
Microbial testing An overview of what it is and what it is for Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the following chapter is published for. Microbial Test Ep.
From dxolkphsc.blob.core.windows.net
Microbial Testing Labs at Reba Hess blog Microbial Test Ep Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the tests are designed primarily to determine whether a substance or preparation complies with an established specification for. This general chapter presents 2 sets of. Microbial Test Ep.
From dxolkphsc.blob.core.windows.net
Microbial Testing Labs at Reba Hess blog Microbial Test Ep it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. the following chapter is published for information. This general chapter presents 2 sets of recommended acceptance criteria for. microbial enumeration tests are quantitative methods to determine a product’s level of contamination with mesophilic bacteria and. the tests are designed primarily. Microbial Test Ep.
From lotioncrafter.com
Microbial Test Kit, 10 Pack Lotioncrafter Microbial Test Ep it aims to facilitate the recognition of pharmacopoeial procedures for microbial enumeration tests by regulatory authorities. This general chapter presents 2 sets of recommended acceptance criteria for. Introduction the tests described hereafter will allow quantitative enumeration of mesophilic bacteria and fungi that may grow. the tests are designed primarily to determine whether a substance or preparation complies with. Microbial Test Ep.