Nitrous Oxide Balanced Equation . Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. Nitric oxide (radical) + dioxygen = nitrogen. it has a linear structure. 2 no + o 2 → 2 no 2. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Resonance structures of nitrogen (i) oxide. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Three resonance structures can be drawn for nitrous oxide.
from www.numerade.com
Nitric oxide (radical) + dioxygen = nitrogen. it has a linear structure. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. 2 no + o 2 → 2 no 2. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Three resonance structures can be drawn for nitrous oxide. Resonance structures of nitrogen (i) oxide. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the.
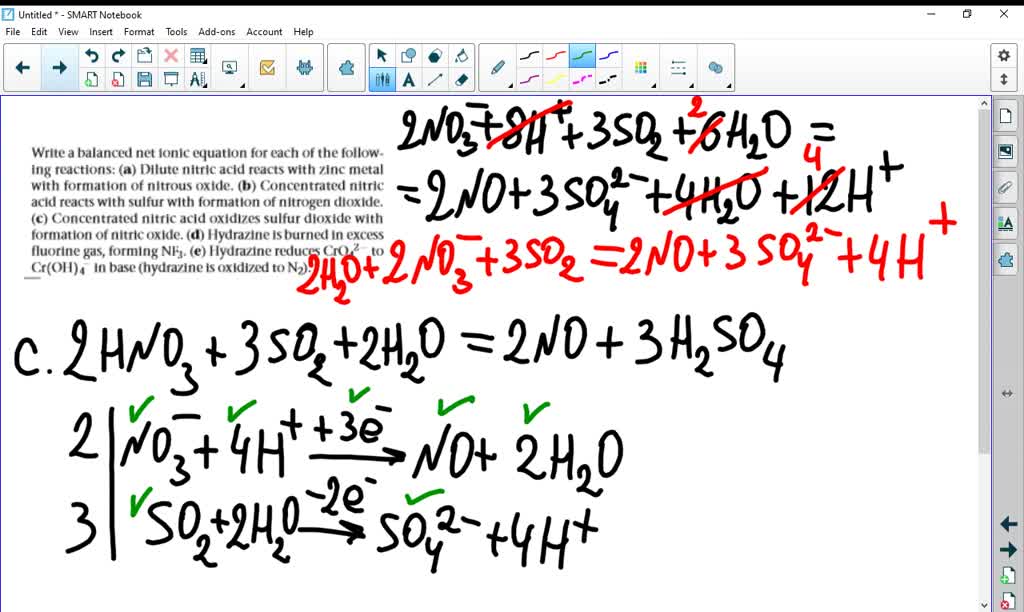
SOLVEDWrite a balanced net ionic equation for each of the following
Nitrous Oxide Balanced Equation Resonance structures of nitrogen (i) oxide. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. Resonance structures of nitrogen (i) oxide. Three resonance structures can be drawn for nitrous oxide. Nitric oxide (radical) + dioxygen = nitrogen. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. it has a linear structure. 2 no + o 2 → 2 no 2. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the.
From www.toppr.com
copper on addition to nitric acid gets converted to gaseous nitric Nitrous Oxide Balanced Equation n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. it has a linear structure. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Multiply the oxidation. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced equation for each of the following processes Nitrous Oxide Balanced Equation Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number. Nitrous Oxide Balanced Equation.
From brainly.in
Balance the equation nitrogen + oxygen > nitrous oxide Brainly.in Nitrous Oxide Balanced Equation Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. Resonance structures of nitrogen (i) oxide. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. 2 no + o 2 → 2. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED Nitrous acid (HNO2) disproportionates in acidic solutionto Nitrous Oxide Balanced Equation 2 no + o 2 → 2 no 2. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. it has. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Nitrous Oxide Balanced Equation Three resonance structures can be drawn for nitrous oxide. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Multiply the. Nitrous Oxide Balanced Equation.
From www.alamy.com
Molecular Model of Nitrous Oxide (N2O) Molecule. Vector Illustration Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Resonance structures of nitrogen (i) oxide. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain. Nitrous Oxide Balanced Equation.
From www.alamy.com
Molecular Model of Nitrous Oxide (N2O) Molecule. Vector Illustration Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Three resonance structures can be drawn for nitrous oxide. 2 no + o 2 → 2 no 2. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Resonance. Nitrous Oxide Balanced Equation.
From www.slideserve.com
PPT Collision Theory & Reaction Mechanisms PowerPoint Presentation Nitrous Oxide Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. Nitric oxide (radical) + dioxygen = nitrogen. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms. Nitrous Oxide Balanced Equation.
From www.toppr.com
2NO N2O2 (fast) N2O2 + H2→ H2O2 + N2 (slow) H2 + H2O2→ 2H2O (fast)For Nitrous Oxide Balanced Equation write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. it has a linear structure. Resonance structures of nitrogen (i) oxide.. Nitrous Oxide Balanced Equation.
From mungfali.com
Balanced Equation For Nitrogen Oxide Nitrous Oxide Balanced Equation n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. . Nitrous Oxide Balanced Equation.
From mungfali.com
Balanced Equation For Nitrogen Oxide Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Resonance structures of nitrogen (i) oxide. Nitric oxide (radical) + dioxygen = nitrogen. 2 no + o 2 → 2 no 2. n2o = n + o is a decomposition reaction where one mole of nitrous oxide. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced net ionic equation for each of the following Nitrous Oxide Balanced Equation Three resonance structures can be drawn for nitrous oxide. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. 2 no + o 2 → 2 no 2. Resonance structures of nitrogen (i) oxide. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite complete balanced halfreactions for (a) reduction of Nitrous Oxide Balanced Equation it has a linear structure. Three resonance structures can be drawn for nitrous oxide. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are). Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED A proposed mechanism for the oxidation of nitric oxide to Nitrous Oxide Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. 2 no + o 2 → 2 no 2. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Nitric oxide. Nitrous Oxide Balanced Equation.
From www.youtube.com
N2O=N2+O2 Balanced EquationNitrous oxide=Nitrogen+Oxygen Balanced Nitrous Oxide Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. 2 no + o 2 → 2 no 2. Three resonance structures can be drawn for nitrous oxide. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite complete balanced halfreactions for (a) oxidation of Nitrous Oxide Balanced Equation write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. it has a linear structure. Three resonance structures can be drawn for nitrous oxide. n2o = n + o is a decomposition reaction where one mole of nitrous. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED Thank you for your help 1. Write the unbalanced, and balanced Nitrous Oxide Balanced Equation write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Three resonance structures can be drawn for nitrous oxide. 2 no + o 2 → 2 no 2. it has a linear structure. the chemical equation described in. Nitrous Oxide Balanced Equation.
From solvedlib.com
STOICHIOMETRYSolving for a reactant using chemical eq… SolvedLib Nitrous Oxide Balanced Equation Nitric oxide (radical) + dioxygen = nitrogen. Three resonance structures can be drawn for nitrous oxide. 2 no + o 2 → 2 no 2. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. the chemical equation described. Nitrous Oxide Balanced Equation.
From www.youtube.com
How to Balance HNO2 + NH3 = NH4NO2 (nitrious acid and ammonia) YouTube Nitrous Oxide Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. 2 no + o 2 → 2 no 2. Three resonance structures can be drawn for nitrous oxide. it has a linear. Nitrous Oxide Balanced Equation.
From slideplayer.com
Stoichiometry Ratios of Combination ppt download Nitrous Oxide Balanced Equation n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Nitric oxide (radical) + dioxygen = nitrogen. Resonance structures. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVEDNitrous oxide (N2 O) has three possible Lewis structures Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. it has a linear structure. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. 2 no + o 2 → 2 no 2. write separate equations for. Nitrous Oxide Balanced Equation.
From www.numerade.com
The mechanism for the reaction of nitrogen dioxide with carbon monoxide Nitrous Oxide Balanced Equation 2 no + o 2 → 2 no 2. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED Dinitrogen oxide commonly called nitrous oxide is used as a Nitrous Oxide Balanced Equation Resonance structures of nitrogen (i) oxide. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. the chemical equation balancer can. Nitrous Oxide Balanced Equation.
From www.alamy.com
Nitric oxide, NO, molecule model and chemical formula. Nitrogen oxide Nitrous Oxide Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. 2 no + o 2 → 2 no 2. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Three resonance. Nitrous Oxide Balanced Equation.
From melscience.com
Methods of synthesis of nitrous oxide MEL Chemistry Nitrous Oxide Balanced Equation Resonance structures of nitrogen (i) oxide. Three resonance structures can be drawn for nitrous oxide. 2 no + o 2 → 2 no 2. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. Multiply the oxidation and reduction equations by. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED 369 of RedoxBalancing Which of the following is the correctly Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Resonance structures of nitrogen (i) oxide. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. 2 no. Nitrous Oxide Balanced Equation.
From oneclass.com
OneClass Name the compound HNO2 (aq). Hydrogen nitrogen dioxide Nitrous Oxide Balanced Equation Nitric oxide (radical) + dioxygen = nitrogen. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles. Nitrous Oxide Balanced Equation.
From brainly.in
Give the balanced equation to prepare Nitric oxide. Which type of oxide Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes. Nitrous Oxide Balanced Equation.
From www.youtube.com
How to Balance C5H9O + O2 = CO2 + H2O YouTube Nitrous Oxide Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Multiply the oxidation and reduction equations by appropriate coefficients so that. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED The mechanism for the reaction of nitrogen dioxide with carbon Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Nitric oxide (radical) + dioxygen = nitrogen. Multiply the oxidation and reduction equations by appropriate coefficients so that both contain the same number of electrons. write separate equations for oxidation and reduction, showing (a) the atom (s). Nitrous Oxide Balanced Equation.
From www.toppr.com
Write balanced chemical equations the following word equationNitrogen Nitrous Oxide Balanced Equation it has a linear structure. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. Three resonance structures can be drawn for nitrous oxide. Resonance structures of nitrogen (i) oxide. Nitric oxide (radical) + dioxygen = nitrogen. the chemical. Nitrous Oxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced halfreaction for the oxidation of gaseous Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Resonance structures of nitrogen (i) oxide. n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. Multiply the oxidation. Nitrous Oxide Balanced Equation.
From dxobpicld.blob.core.windows.net
What Is The Formula For Nitric Oxide at Camper blog Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. write separate equations for oxidation and reduction, showing (a) the atom (s) that is (are) oxidized and reduced and (b) the number of electrons accepted or donated by each. Resonance structures of nitrogen (i) oxide. it. Nitrous Oxide Balanced Equation.
From testbook.com
Nitrous Oxide Definition, Structure, Formula, Properties, Uses Nitrous Oxide Balanced Equation n2o = n + o is a decomposition reaction where one mole of nitrous oxide [n 2 o] decomposes into two moles of nitrogen [n] and one mole. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. the chemical equation balancer can find coefficients to. Nitrous Oxide Balanced Equation.
From www.studypool.com
SOLUTION 1) The Nitric oxide shown in the balanced equation is Nitrous Oxide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. 2 no + o 2 → 2 no 2. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Resonance structures of nitrogen (i) oxide. Nitric oxide (radical) +. Nitrous Oxide Balanced Equation.