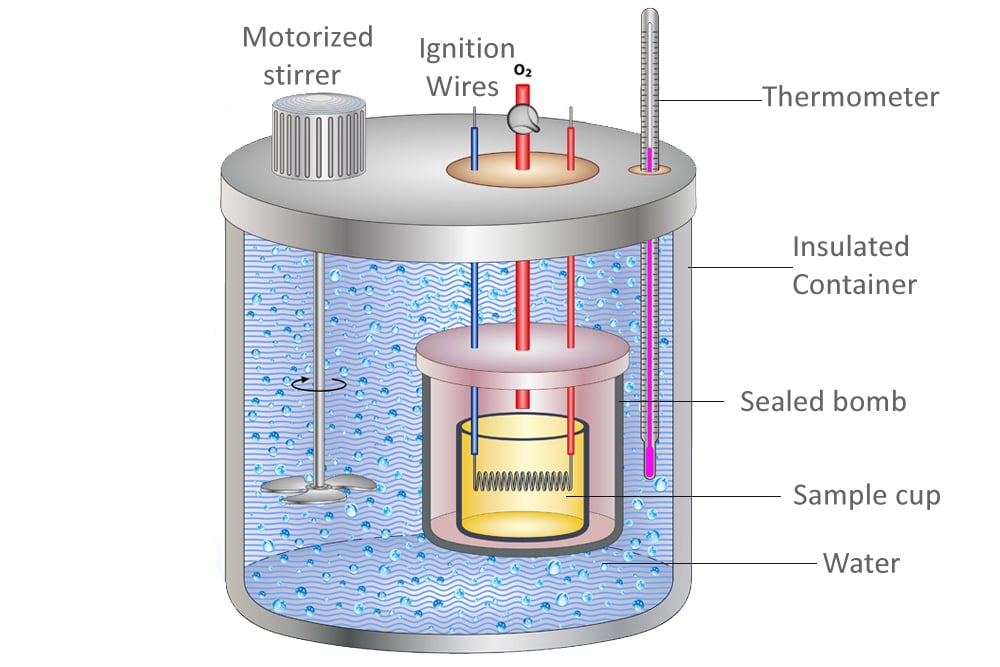
What Is A Calorimeter Made Of . A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Chemists use calorimetry to determine the. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
from www.scienceabc.com
It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
Molar Heat Capacity Definition, Formula, Equation, Calculation
What Is A Calorimeter Made Of Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 What Is A Calorimeter Made Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic reaction occurs in solution in. What Is A Calorimeter Made Of.
From scienceinfo.com
Calorimeter Definition, Types and Uses What Is A Calorimeter Made Of Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good conductors of electricity. What Is A Calorimeter Made Of.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used for heat measurements necessary for calorimetry. Chemists use calorimetry to determine the. Calorimeter, device for measuring the heat developed. What Is A Calorimeter Made Of.
From saylordotorg.github.io
Calorimetry What Is A Calorimeter Made Of Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. What Is A Calorimeter Made Of.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Apply the first law of thermodynamics to calorimetry. It mainly consists of a metallic vessel made of materials which are good conductors. What Is A Calorimeter Made Of.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Is A Calorimeter Made Of Apply the first law of thermodynamics to calorimetry. Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in. What Is A Calorimeter Made Of.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube What Is A Calorimeter Made Of Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the.. What Is A Calorimeter Made Of.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of. What Is A Calorimeter Made Of.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is A Calorimeter Made Of Chemists use calorimetry to determine the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a field of thermochemistry that. What Is A Calorimeter Made Of.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Chemists use calorimetry to determine the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat.. What Is A Calorimeter Made Of.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter What Is A Calorimeter Made Of Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. What Is A Calorimeter Made Of.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning What Is A Calorimeter Made Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. It mainly consists of a metallic vessel made of materials which are good conductors of electricity. What Is A Calorimeter Made Of.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Calorimetry is a field of thermochemistry that measures. What Is A Calorimeter Made Of.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is A Calorimeter Made Of It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Chemists use calorimetry to determine the. Compare heat flow from hot to cold objects in an. What Is A Calorimeter Made Of.
From courses.lumenlearning.com
Calorimetry Chemistry I What Is A Calorimeter Made Of A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during. What Is A Calorimeter Made Of.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry What Is A Calorimeter Made Of Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. A calorimeter is a device used for heat measurements necessary for calorimetry. Apply the first law. What Is A Calorimeter Made Of.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Chemists use calorimetry to determine the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Calorimetry is a field of thermochemistry that measures. What Is A Calorimeter Made Of.
From www.animalia-life.club
Calorimeter Diagram What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a field of thermochemistry that measures the amount. What Is A Calorimeter Made Of.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is A Calorimeter Made Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Chemists use calorimetry to determine the. Calorimeter, device for measuring the heat developed during. What Is A Calorimeter Made Of.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure What Is A Calorimeter Made Of Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when. What Is A Calorimeter Made Of.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Is A Calorimeter Made Of It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. Calorimetry is. What Is A Calorimeter Made Of.
From 2012books.lardbucket.org
Calorimetry What Is A Calorimeter Made Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic. What Is A Calorimeter Made Of.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Is A Calorimeter Made Of For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. Compare heat flow. What Is A Calorimeter Made Of.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. What Is A Calorimeter Made Of.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 What Is A Calorimeter Made Of A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.. What Is A Calorimeter Made Of.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary What Is A Calorimeter Made Of Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists. What Is A Calorimeter Made Of.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog What Is A Calorimeter Made Of Apply the first law of thermodynamics to calorimetry. Chemists use calorimetry to determine the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. What Is A Calorimeter Made Of.
From www.youtube.com
Constructing a Calorimeter YouTube What Is A Calorimeter Made Of A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Chemists use calorimetry to determine the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Apply the first law of thermodynamics to calorimetry. Calorimetry is. What Is A Calorimeter Made Of.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. What Is A Calorimeter Made Of.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure What Is A Calorimeter Made Of Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a field of thermochemistry that measures the amount of heat. What Is A Calorimeter Made Of.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Is A Calorimeter Made Of A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimeter, device for measuring. What Is A Calorimeter Made Of.
From loejdmoqi.blob.core.windows.net
Calorimeter And The Function at Judith Stanton blog What Is A Calorimeter Made Of For example, when an exothermic reaction occurs in solution in a calorimeter, the. Chemists use calorimetry to determine the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Calorimeter, device for measuring the heat developed during. What Is A Calorimeter Made Of.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is A Calorimeter Made Of Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Chemists use calorimetry to determine the. A calorimeter is a device used. What Is A Calorimeter Made Of.
From byjus.com
The calorimeter is commonly made up of……..because it has……. What Is A Calorimeter Made Of Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. A calorimeter is a device. What Is A Calorimeter Made Of.
From loerzgmcm.blob.core.windows.net
How To Make A Homemade Calorimeter at Norma Mercer blog What Is A Calorimeter Made Of It mainly consists of a metallic vessel made of materials which are good conductors of electricity such. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used for heat measurements necessary for calorimetry. Chemists use calorimetry to determine the. Apply the first law of thermodynamics to calorimetry. A calorimeter is a. What Is A Calorimeter Made Of.