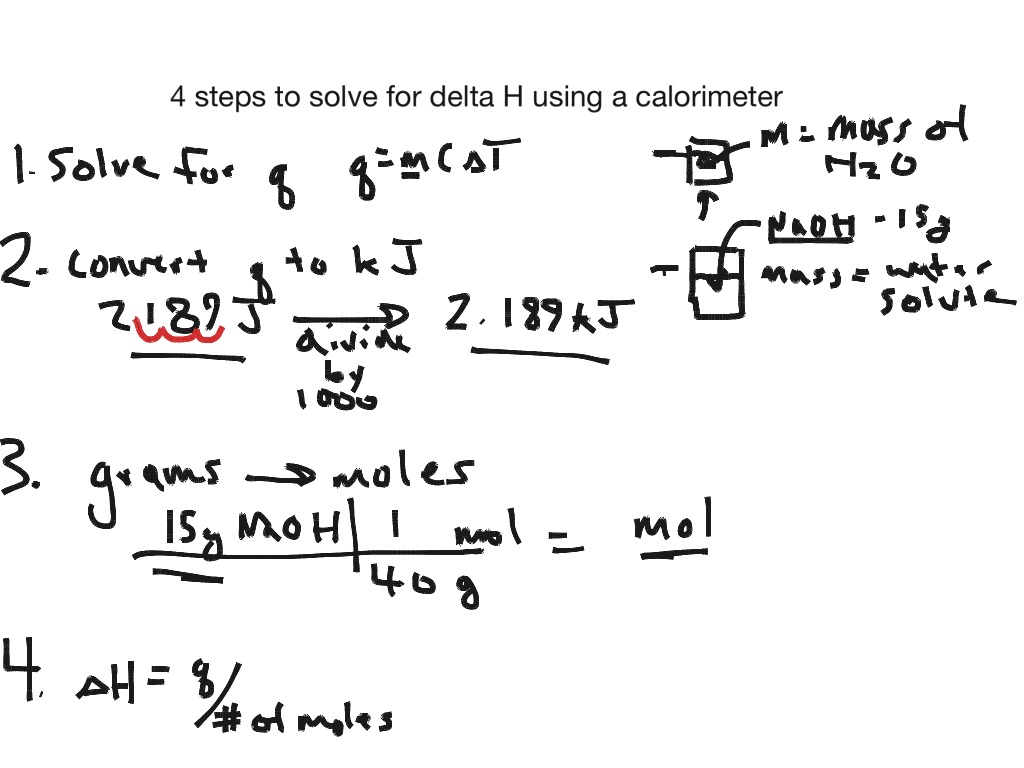
Calorimetry Formula Reaction . Q is the heat energy. From this temperature change, we can calculate the energy change. The equation to calculate enthalpy changes from temperature changes is: One technique we can use to measure the amount of heat involved in a chemical or physical process is known. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. M is the mass of the. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Q = m × c × δt. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. measuring heat flow.
from www.showme.com
From this temperature change, we can calculate the energy change. The equation to calculate enthalpy changes from temperature changes is: reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. M is the mass of the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. Q = m × c × δt. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Q is the heat energy. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical.
4 steps for solving calorimetry problems Science, Chemistry ShowMe
Calorimetry Formula Reaction The equation to calculate enthalpy changes from temperature changes is: calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. From this temperature change, we can calculate the energy change. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. The equation to calculate enthalpy changes from temperature changes is: reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. Q = m × c × δt. measuring heat flow. M is the mass of the. Q is the heat energy.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Formula Reaction in calorimetry, we measure the temperature change of a substance that surrounds a reaction. Q = m × c × δt. Q is the heat energy. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. measuring heat flow. From this temperature change, we can. Calorimetry Formula Reaction.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Calorimetry Formula Reaction reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. Q = m × c × δt. measuring heat flow. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. M is the mass of the. One technique we can use to. Calorimetry Formula Reaction.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Formula Reaction measuring heat flow. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. Calorimetry Formula Reaction.
From www.slideserve.com
PPT A calorimeter is used to measure the amount of heat absorbed or Calorimetry Formula Reaction calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. Q is the heat energy. One technique we can use to measure the amount of heat involved in a chemical or physical process. Calorimetry Formula Reaction.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Formula Reaction one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. measuring heat flow. Q is the heat energy. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Q = m × c × δt. . Calorimetry Formula Reaction.
From www.slideshare.net
Calorimetry Calorimetry Formula Reaction Q = m × c × δt. M is the mass of the. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Q is the heat energy. reaction calorimetry is. Calorimetry Formula Reaction.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Formula Reaction measuring heat flow. Q = m × c × δt. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calculate heat, temperature change, and. Calorimetry Formula Reaction.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Formula Reaction Q = m × c × δt. Q is the heat energy. From this temperature change, we can calculate the energy change. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. one technique we can use to measure the amount of heat. Calorimetry Formula Reaction.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Formula Reaction From this temperature change, we can calculate the energy change. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. measuring heat flow. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. One technique we can use to measure the amount. Calorimetry Formula Reaction.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Formula Reaction M is the mass of the. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The equation to calculate enthalpy changes from temperature changes is: measuring heat flow. Q = m × c × δt. One technique we can use to measure the amount of. Calorimetry Formula Reaction.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID197266 Calorimetry Formula Reaction calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Q = m × c × δt. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a. Calorimetry Formula Reaction.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimetry Formula Reaction From this temperature change, we can calculate the energy change. Q is the heat energy. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. M is the mass of the. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calorimetry Formula Reaction.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Formula Reaction one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From this temperature change, we can calculate the energy change. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. . Calorimetry Formula Reaction.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Formula Reaction M is the mass of the. Q is the heat energy. From this temperature change, we can calculate the energy change. The equation to calculate enthalpy changes from temperature changes is: measuring heat flow. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique. Calorimetry Formula Reaction.
From www.youtube.com
Calorimetry of H2O2 Reactions Intro & Theory YouTube Calorimetry Formula Reaction From this temperature change, we can calculate the energy change. The equation to calculate enthalpy changes from temperature changes is: Q = m × c × δt. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other. Calorimetry Formula Reaction.
From www.youtube.com
Reaction Calorimetry Example 1 YouTube Calorimetry Formula Reaction One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Q is the heat energy. The equation to calculate enthalpy changes from temperature changes is: calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. one technique we can use to. Calorimetry Formula Reaction.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Formula Reaction Q = m × c × δt. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. The equation to calculate enthalpy changes from temperature changes is: calculate. Calorimetry Formula Reaction.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Calorimetry Formula Reaction Q = m × c × δt. measuring heat flow. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. The equation to calculate enthalpy changes from temperature changes is: Q is the heat energy. one technique we can use to measure. Calorimetry Formula Reaction.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Formula Reaction The equation to calculate enthalpy changes from temperature changes is: measuring heat flow. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From this temperature change, we can calculate the energy change. in calorimetry, we measure the temperature change of a substance that surrounds. Calorimetry Formula Reaction.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Formula Reaction Q is the heat energy. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. measuring heat flow. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. One technique we can use to measure the amount of heat involved in a chemical or physical process. Calorimetry Formula Reaction.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Formula Reaction M is the mass of the. measuring heat flow. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. in this article, you will learn about calorimetry and calorimeters,. Calorimetry Formula Reaction.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube Calorimetry Formula Reaction calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Q = m × c × δt. measuring heat flow. Q is the heat energy. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. From this temperature change, we can calculate. Calorimetry Formula Reaction.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Calorimetry Formula Reaction in calorimetry, we measure the temperature change of a substance that surrounds a reaction. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. measuring heat flow. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Calorimetry Formula Reaction.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimetry Formula Reaction Q is the heat energy. measuring heat flow. From this temperature change, we can calculate the energy change. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. Q = m × c ×. Calorimetry Formula Reaction.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Calorimetry Formula Reaction One technique we can use to measure the amount of heat involved in a chemical or physical process is known. The equation to calculate enthalpy changes from temperature changes is: M is the mass of the. From this temperature change, we can calculate the energy change. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. Calorimetry Formula Reaction.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Calorimetry Formula Reaction in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. measuring heat flow. M is the mass of the. Q is the heat energy. one technique we can use to measure the amount of heat involved in a chemical or physical process. Calorimetry Formula Reaction.
From worksheetmediadwaum.z14.web.core.windows.net
How To Calculate Calorimetry Problems Calorimetry Formula Reaction one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. measuring heat flow. Q = m × c × δt.. Calorimetry Formula Reaction.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Calorimetry Formula Reaction The equation to calculate enthalpy changes from temperature changes is: one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. M is the mass of the. Q = m × c × δt. Q is the heat energy. From this temperature change, we can calculate the energy. Calorimetry Formula Reaction.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Formula Reaction The equation to calculate enthalpy changes from temperature changes is: in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. one technique we can use. Calorimetry Formula Reaction.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry Formula Reaction The equation to calculate enthalpy changes from temperature changes is: reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. From this temperature change, we can calculate the energy change. . Calorimetry Formula Reaction.
From ayanahcristien.blogspot.com
20+ Calculating Heat Of Reaction From ConstantPressure Calorimetry Calorimetry Formula Reaction measuring heat flow. in calorimetry, we measure the temperature change of a substance that surrounds a reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a.. Calorimetry Formula Reaction.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Calorimetry Formula Reaction M is the mass of the. From this temperature change, we can calculate the energy change. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or other chemical. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. The equation to calculate enthalpy changes from. Calorimetry Formula Reaction.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Formula Reaction M is the mass of the. Q = m × c × δt. The equation to calculate enthalpy changes from temperature changes is: One technique we can use to measure the amount of heat involved in a chemical or physical process is known. reaction calorimetry is used to evaluate the molar integral reaction enthalpy δhm(rxn) of a reaction or. Calorimetry Formula Reaction.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Calorimetry Formula Reaction M is the mass of the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. The equation to calculate enthalpy changes from temperature changes is: measuring heat flow. Q is. Calorimetry Formula Reaction.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Formula Reaction calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Q = m × c × δt. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical. reaction calorimetry is used to evaluate the molar. Calorimetry Formula Reaction.