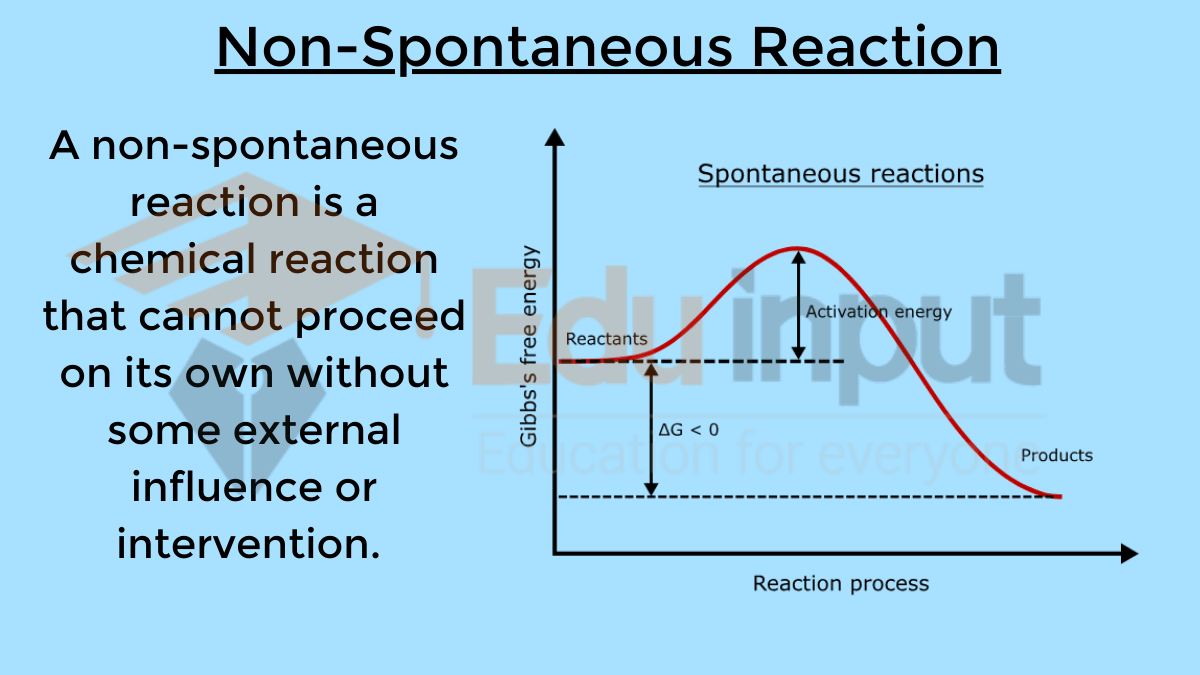
Catalyst Does Not Catalyse Non-Spontaneous Reaction . Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). It assumes that you are already familiar with basic ideas about the collision. Provided that the catalyst can catalyse the forward. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts do not appear in the overall chemical equation for a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. They do not appear in the reaction’s net equation and are not consumed during the. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. A catalyst provides an alternative.
from eduinput.com
this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision. A catalyst provides an alternative. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the. catalysts do not appear in the overall chemical equation for a reaction. catalysts participate in a chemical reaction and increase its rate. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). Provided that the catalyst can catalyse the forward.
NonSpontaneous ReactionsThermochemistry, and Examples
Catalyst Does Not Catalyse Non-Spontaneous Reaction Provided that the catalyst can catalyse the forward. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). Provided that the catalyst can catalyse the forward. catalysts participate in a chemical reaction and increase its rate. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts do not appear in the overall chemical equation for a reaction. It assumes that you are already familiar with basic ideas about the collision. A catalyst provides an alternative. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes.
From www.differencebetween.com
Difference Between Catalytic and Non Catalytic Reaction Compare the Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). Provided that. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.slideserve.com
PPT Jöns Jacob Berzelius Born 20 August 1779 Väversunda, Östergötland Catalyst Does Not Catalyse Non-Spontaneous Reaction since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. It assumes that you are already familiar with basic ideas about the collision.. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Does Not Catalyse Non-Spontaneous Reaction since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Provided that the catalyst can catalyse the forward. catalysts do not appear in the overall chemical equation for a reaction.. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.chemistrylearner.com
Spontaneous and Nonspontaneous Reaction Definition and Examples Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. Provided that the catalyst can catalyse the forward. They do not appear in the reaction’s net equation and are not consumed during the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It assumes that you are. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From eduinput.com
Difference between Spontaneous and NonSpontaneous Reactions Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). It assumes. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.toppr.com
For a non spontaneous reaction, at 30^oC , Δ H> 0 and Δ S> 0 . The Catalyst Does Not Catalyse Non-Spontaneous Reaction since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision. Catalysts can be homogenous (in the same phase. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From eduinput.com
10 Examples of Spontaneous Reactions Catalyst Does Not Catalyse Non-Spontaneous Reaction They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From printablelibvera.z13.web.core.windows.net
Labeled Enzyme Reaction Graph Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. It assumes that you are already familiar with basic ideas about the collision. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). Provided that the catalyst can catalyse the forward. this page describes and explains the. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From studybeglerbegs.z4.web.core.windows.net
How Do Enzymes Catalyze Reactions Catalyst Does Not Catalyse Non-Spontaneous Reaction Provided that the catalyst can catalyse the forward. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). catalysts do not appear in the overall chemical equation for a reaction. A catalyst provides an alternative. They do not appear in the reaction’s net equation and are not consumed during the.. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Does Not Catalyse Non-Spontaneous Reaction this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts do not appear in the overall chemical equation for a reaction. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). since the potential energy surface does not depend on. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Does Not Catalyse Non-Spontaneous Reaction this page describes and explains the way that adding a catalyst affects the rate of a reaction. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts participate in a chemical reaction and increase its rate. It assumes that you are already familiar with basic ideas. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.expii.com
Spontaneous and Nonspontaneous Reactions — Overview Expii Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts participate in a chemical reaction and increase its rate. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). A catalyst provides an alternative. It assumes that you. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From eduinput.com
10 Examples of NonSpontaneous Reactions Catalyst Does Not Catalyse Non-Spontaneous Reaction Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). catalysts participate in a chemical reaction and increase its rate. Provided that the catalyst can catalyse the forward. They do not appear in the reaction’s net equation and are not consumed during the. catalysts do not appear in the. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.slideserve.com
PPT Metabolism PowerPoint Presentation, free download ID3118365 Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. Provided that the catalyst can catalyse the forward. this page describes and explains the way that adding a catalyst affects the rate of a reaction. They do not appear in the reaction’s net equation and are not consumed during the. catalysts affect the rate of. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst provides an alternative. Catalysts can be homogenous (in. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.etutorworld.com
Spontaneous and NonSpontaneous Processes Catalyst Does Not Catalyse Non-Spontaneous Reaction since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts do not appear in the overall chemical equation for a reaction. catalysts participate in a chemical reaction and increase its rate. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From brainly.in
Difference between spontaneous and non spontaneous reactions? Brainly.in Catalyst Does Not Catalyse Non-Spontaneous Reaction It assumes that you are already familiar with basic ideas about the collision. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. They do not appear in the reaction’s net equation and are not consumed during the. catalysts do not appear in the overall chemical equation for. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.slideserve.com
PPT Entropy and Free Energy PowerPoint Presentation, free download Catalyst Does Not Catalyse Non-Spontaneous Reaction They do not appear in the reaction’s net equation and are not consumed during the. this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst provides an alternative. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). catalysts affect. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From exoshlpnk.blob.core.windows.net
Catalyst In The Human Body Are Called at Harold Levy blog Catalyst Does Not Catalyse Non-Spontaneous Reaction since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts do not appear in the overall chemical equation for a reaction. They do not appear in the reaction’s net equation and are not consumed during the. A catalyst provides an alternative. Catalysts can be homogenous (in the. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From eduinput.com
NonSpontaneous ReactionsThermochemistry, and Examples Catalyst Does Not Catalyse Non-Spontaneous Reaction A catalyst provides an alternative. catalysts participate in a chemical reaction and increase its rate. catalysts do not appear in the overall chemical equation for a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.slideserve.com
PPT Chapter 16 Reaction Energy PowerPoint Presentation, free download Catalyst Does Not Catalyse Non-Spontaneous Reaction since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts participate in a chemical reaction and increase its rate. It assumes that you are already familiar with basic ideas about the collision. this page describes and explains the way that adding a catalyst affects the rate. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From schematicbraginamh.z4.web.core.windows.net
Energy Diagram For Chemical Reaction Catalyst Does Not Catalyse Non-Spontaneous Reaction A catalyst provides an alternative. It assumes that you are already familiar with basic ideas about the collision. catalysts do not appear in the overall chemical equation for a reaction. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). since the potential energy surface does not depend on. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From 2012books.lardbucket.org
Catalysis Catalyst Does Not Catalyse Non-Spontaneous Reaction It assumes that you are already familiar with basic ideas about the collision. Provided that the catalyst can catalyse the forward. catalysts participate in a chemical reaction and increase its rate. catalysts do not appear in the overall chemical equation for a reaction. this page describes and explains the way that adding a catalyst affects the rate. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.youtube.com
What are non spontaneous reactions? YouTube Catalyst Does Not Catalyse Non-Spontaneous Reaction A catalyst provides an alternative. catalysts participate in a chemical reaction and increase its rate. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It assumes that you. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID239015 Catalyst Does Not Catalyse Non-Spontaneous Reaction this page describes and explains the way that adding a catalyst affects the rate of a reaction. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts do not appear in the overall chemical equation for a reaction. catalysts affect the rate of a chemical. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.youtube.com
NonSpontaneous Reaction (Example) YouTube Catalyst Does Not Catalyse Non-Spontaneous Reaction It assumes that you are already familiar with basic ideas about the collision. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts participate in a chemical reaction. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). this page describes and explains the way that adding a catalyst affects the rate of a reaction. since the potential energy surface does not depend on. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.chemistrylearner.com
Spontaneous and Nonspontaneous Reaction Definition and Examples Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. It assumes that you are already familiar with basic ideas about the collision. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. They do not appear in the reaction’s net equation and are not consumed. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From zymvol.com
All you need to know about enzymes Zymvol Catalyst Does Not Catalyse Non-Spontaneous Reaction They do not appear in the reaction’s net equation and are not consumed during the. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts participate in a chemical reaction and increase its rate. Provided that the catalyst can catalyse the forward. since the potential energy surface does not. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.sciencemotive.com
HalfLife of Reactions in Chemical ScienceMotive Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. since. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.etutorworld.com
Spontaneous and NonSpontaneous Processes Catalyst Does Not Catalyse Non-Spontaneous Reaction catalysts do not appear in the overall chemical equation for a reaction. It assumes that you are already familiar with basic ideas about the collision. Provided that the catalyst can catalyse the forward. They do not appear in the reaction’s net equation and are not consumed during the. since the potential energy surface does not depend on the. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From eduinput.com
Spontaneous ReactionsThermochemistry, and Examples Catalyst Does Not Catalyse Non-Spontaneous Reaction It assumes that you are already familiar with basic ideas about the collision. since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. They do not appear in the reaction’s net equation and are not consumed during the. Catalysts can be homogenous (in the same phase as the reactants). Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.slideserve.com
PPT Entropy and Free Energy PowerPoint Presentation, free download Catalyst Does Not Catalyse Non-Spontaneous Reaction A catalyst provides an alternative. catalysts do not appear in the overall chemical equation for a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It assumes that you are already familiar with basic ideas about the collision. Catalysts can be homogenous (in the same phase as the. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst Does Not Catalyse Non-Spontaneous Reaction It assumes that you are already familiar with basic ideas about the collision. A catalyst provides an alternative. catalysts participate in a chemical reaction and increase its rate. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Provided that the catalyst can catalyse the forward. since the potential energy. Catalyst Does Not Catalyse Non-Spontaneous Reaction.
From www.differencebetween.com
Difference Between Catalytic and Non Catalytic Reaction Compare the Catalyst Does Not Catalyse Non-Spontaneous Reaction since the potential energy surface does not depend on the direction of the reaction the answer is a conditional yes. A catalyst provides an alternative. Provided that the catalyst can catalyse the forward. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts participate in a chemical reaction and. Catalyst Does Not Catalyse Non-Spontaneous Reaction.