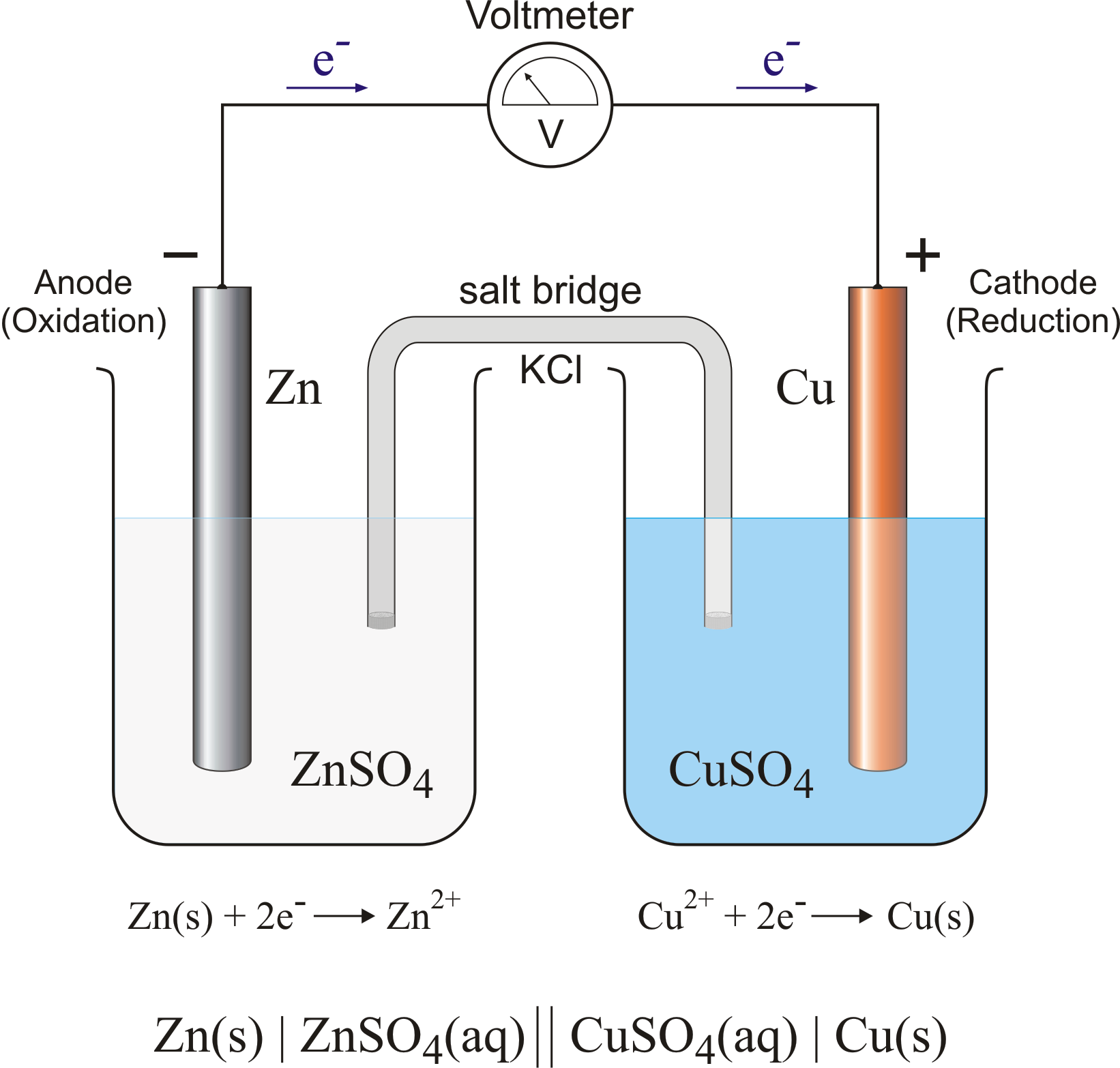
A Galvanic Cell Is At Equilibrium When . In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. Consider the setup zn|zn2+||cu2+|cu, where. What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's.
from glossary.periodni.com
In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. Consider the setup zn|zn2+||cu2+|cu, where. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell.
Bunsenov članak Chemistry Dictionary & Glossary
A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Consider the setup zn|zn2+||cu2+|cu, where. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction.
From solvedlib.com
Calculate the equilibrium constant of a galvanic cell… SolvedLib A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. Consider the setup zn|zn2+||cu2+|cu, where. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From chem.eduinsightful.com
Galvanic Cells Decoded Igniting a Spark of Knowledge A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. Consider the setup zn|zn2+||cu2+|cu, where. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From glossary.periodni.com
Bunsenov članak Chemistry Dictionary & Glossary A Galvanic Cell Is At Equilibrium When Consider the setup zn|zn2+||cu2+|cu, where. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The nernst equation calculates electrochemical cell potential from standard cell. A Galvanic Cell Is At Equilibrium When.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when. A Galvanic Cell Is At Equilibrium When.
From www.youtube.com
Galvanic cell or Voltaic cell Definition ,Construction and Working A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. Consider the setup zn|zn2+||cu2+|cu, where. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in. A Galvanic Cell Is At Equilibrium When.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two. A Galvanic Cell Is At Equilibrium When.
From www.youtube.com
18.8 Equilibrium Constants for Galvanic Cells YouTube A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two. A Galvanic Cell Is At Equilibrium When.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The redox reaction is spontaneously approaching. A Galvanic Cell Is At Equilibrium When.
From www.clutchprep.com
Galvanic Cell Chemistry Video Clutch Prep A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Consider the setup zn|zn2+||cu2+|cu, where. What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Consider the setup zn|zn2+||cu2+|cu, where. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From www.numerade.com
SOLVEDEcell =0.010 V for a galvanic cell with this reaction at 25^∘ C A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. Consider the setup zn|zn2+||cu2+|cu, where. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From www.numerade.com
SOLVED Data Table R Ag Gibb Calculated Force EMF Energy AG Galvanic A Galvanic Cell Is At Equilibrium When Consider the setup zn|zn2+||cu2+|cu, where. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The. A Galvanic Cell Is At Equilibrium When.
From www.chegg.com
Solved A galvanic cell is at equilibrium (no current flow), A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Consider the setup zn|zn2+||cu2+|cu, where. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From www.toppr.com
The standard emf of galvanic cell involving 3 moles of electrons in its A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when. A Galvanic Cell Is At Equilibrium When.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in. A Galvanic Cell Is At Equilibrium When.
From www.toppr.com
What will be standard cell potential of galvanic cell with the A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Consider the setup zn|zn2+||cu2+|cu, where. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From www.nanoscience.com
Electrochemistry Nanoscience Instruments A Galvanic Cell Is At Equilibrium When Consider the setup zn|zn2+||cu2+|cu, where. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode. A Galvanic Cell Is At Equilibrium When.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Consider the setup zn|zn2+||cu2+|cu, where. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode. A Galvanic Cell Is At Equilibrium When.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. Consider the setup zn|zn2+||cu2+|cu, where. In a galvanic cell, current is produced when electrons flow externally through the circuit from the. A Galvanic Cell Is At Equilibrium When.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 110 A Galvanic Cell Is At Equilibrium When In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. What exactly happens at the equilibrium. A Galvanic Cell Is At Equilibrium When.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. Consider the setup zn|zn2+||cu2+|cu, where. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts A Galvanic Cell Is At Equilibrium When In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell. A Galvanic Cell Is At Equilibrium When.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. Consider the setup zn|zn2+||cu2+|cu, where. What exactly happens at the equilibrium. A Galvanic Cell Is At Equilibrium When.
From biochemreview.weebly.com
Galvanic cell BIOChemReview A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two. A Galvanic Cell Is At Equilibrium When.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. Consider the setup zn|zn2+||cu2+|cu, where. The nernst equation calculates electrochemical cell. A Galvanic Cell Is At Equilibrium When.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts A Galvanic Cell Is At Equilibrium When The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because. A Galvanic Cell Is At Equilibrium When.
From www.youtube.com
Daniell_Galvanic_Voltaic Cell 👉 Salt Bridge 👉 Equilibrium constant K A Galvanic Cell Is At Equilibrium When In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. The redox reaction is spontaneously approaching. A Galvanic Cell Is At Equilibrium When.
From www.coursehero.com
[Solved] 1. In one halfcell of a galvanic cell, a piece of Pt dipped A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because. A Galvanic Cell Is At Equilibrium When.
From www.numerade.com
SOLVEDWhat is the cell potential of a galvanic cell when the cell A Galvanic Cell Is At Equilibrium When In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. The redox reaction is spontaneously approaching. A Galvanic Cell Is At Equilibrium When.
From www.numerade.com
SOLVED Consider the following galvanic cell (10 points) Cma 54 Write A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. In a galvanic cell, current is produced when. A Galvanic Cell Is At Equilibrium When.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle A Galvanic Cell Is At Equilibrium When Consider the setup zn|zn2+||cu2+|cu, where. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when electrons flow externally through the circuit from the. A Galvanic Cell Is At Equilibrium When.
From www.scribd.com
Electrochemical Equilibrium Galvanic Cells Produce Electric Current A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. What exactly happens at the equilibrium. A Galvanic Cell Is At Equilibrium When.
From www.nagwa.com
Question Video Selecting the Correct Anode and Cathode Equilibrium A Galvanic Cell Is At Equilibrium When The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. What exactly happens at the equilibrium state of a galvanic cell redox reaction. Consider the setup zn|zn2+||cu2+|cu, where. In a galvanic. A Galvanic Cell Is At Equilibrium When.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts A Galvanic Cell Is At Equilibrium When In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. What exactly happens at the equilibrium. A Galvanic Cell Is At Equilibrium When.
From www.studocu.com
Chemistry Galvanic Cell10 3.4 Theory of Chemical Equilibrium The A Galvanic Cell Is At Equilibrium When What exactly happens at the equilibrium state of a galvanic cell redox reaction. The redox reaction is spontaneously approaching equilibrium, and as the reaction proceeds, electrons flow within the cell. The nernst equation calculates electrochemical cell potential from standard cell potential, the gas constant, absolute temperature, number of moles of electrons, faraday's. Consider the setup zn|zn2+||cu2+|cu, where. In a galvanic. A Galvanic Cell Is At Equilibrium When.