Bromine Atom In The Ground State . Add an electron to the anion. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. That is, we follow the three important rules:. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the differing orbitals and hybridization,. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps:
from www.jahaneshimi.com
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in an atom, to the differing orbitals and hybridization,. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Add an electron to the anion.
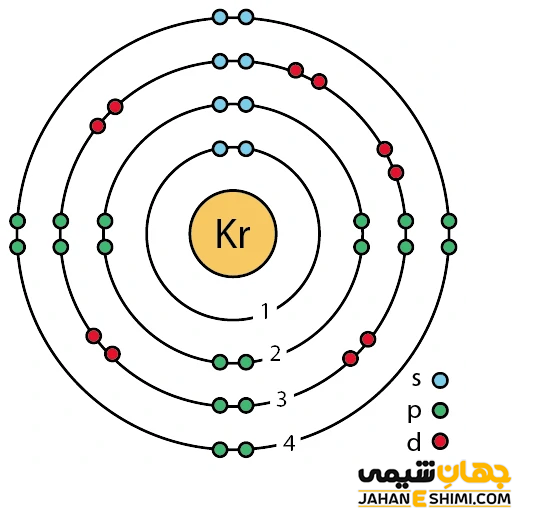
عنصر کریپتون چیست؟ درباره کاربرد کریپتون چه می دانید؟ جهان شیمی فیزیک
Bromine Atom In The Ground State Add an electron to the anion. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the differing orbitals and hybridization,. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Add an electron to the anion. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. That is, we follow the three important rules:.
From ar.inspiredpencil.com
Lewis Dot Structure For Boron Bromine Atom In The Ground State From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. That is, we follow the three important rules:. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: The way we designate electronic configurations for cations and anions. Bromine Atom In The Ground State.
From www.numerade.com
the groundstate electronic configurations of the Bromine Atom In The Ground State That is, we follow the three important rules:. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: The way. Bromine Atom In The Ground State.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The way we designate electronic configurations for cations and anions is. Bromine Atom In The Ground State.
From www.chegg.com
Solved Write the electron configuration for each element in Bromine Atom In The Ground State Add an electron to the anion. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. From the electrons in an atom, to the differing orbitals and hybridization,. The. Bromine Atom In The Ground State.
From www.chegg.com
Solved 6. An electron is in the 1s ground state of a tritium Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Total number of protons in the nucleus is called. Bromine Atom In The Ground State.
From www.toppr.com
If bromine atom is available in the form of, say, two isotopes 35^79Br Bromine Atom In The Ground State From the electrons in an atom, to the differing orbitals and hybridization,. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. That is, we follow the three important rules:. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the. Bromine Atom In The Ground State.
From www.toppr.com
A photon of energy 12.1 eV absorbed by the Hą atom in the ground state Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the differing orbitals and hybridization,. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. That is,. Bromine Atom In The Ground State.
From carisca.github.io
Cr2+ Ground State Electron Configuration Electron Configurations Bromine Atom In The Ground State Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in an atom, to the differing orbitals and hybridization,. That is, we follow the. Bromine Atom In The Ground State.
From www.klipartz.com
Aufbau principio molecular orbital configuración electrónica bromo Bromine Atom In The Ground State That is, we follow the three important rules:. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. If an atom has lost or gained. Bromine Atom In The Ground State.
From www.nagwa.com
Question Video Determining the Figure That Represents an ExcitedState Bromine Atom In The Ground State Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the differing. Bromine Atom In The Ground State.
From abstractor-9fvm.blogspot.com
Numero Atomico De Germanio abstractor Bromine Atom In The Ground State Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Ground state electron configurations are. Bromine Atom In The Ground State.
From byjus.com
On absorbing light of wavelength 3800 Å, bromine molecule undergoes Bromine Atom In The Ground State If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties.. Bromine Atom In The Ground State.
From www.chegg.com
Solved Write the complete groundstate electron Bromine Atom In The Ground State Fundamentally, understanding electron configuration leads to an understanding of the periodic table. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: The way we designate electronic configurations. Bromine Atom In The Ground State.
From nghenhansu.edu.vn
Top 94+ Images How Many Protons, Electrons, And Neutrons Are In An Atom Bromine Atom In The Ground State The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in an atom, to the differing orbitals and hybridization,. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Add an electron to the anion. From the. Bromine Atom In The Ground State.
From rajibrajisha.blogspot.com
7+ bromine bohr diagram RajibRajisha Bromine Atom In The Ground State If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in. Bromine Atom In The Ground State.
From exonchlhy.blob.core.windows.net
What Is The Complete Ground State Electron Configuration For The Bromine Atom In The Ground State Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: The ground state electron configuration of ground. Bromine Atom In The Ground State.
From www.alamy.com
Element bromine hires stock photography and images Alamy Bromine Atom In The Ground State If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. Fundamentally, understanding electron configuration leads to an understanding of. Bromine Atom In The Ground State.
From www.youtube.com
Super Trick Electronic configuration of Bromine Bromine electronic Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. Total number of protons in. Bromine Atom In The Ground State.
From www.chegg.com
Solved tory to do it. Write the groundstate electron Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. That is, we follow the three important rules:. Total number of protons in the nucleus is called the atomic number of the atom and is given the. Bromine Atom In The Ground State.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom In The Ground State That is, we follow the three important rules:. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. From the electrons in an atom, to the differing orbitals and hybridization,. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The way we designate electronic configurations for cations and anions is essentially. Bromine Atom In The Ground State.
From exoqqckld.blob.core.windows.net
Bromine Fluoride Ion at Frank Clemons blog Bromine Atom In The Ground State From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. That is, we. Bromine Atom In The Ground State.
From kunduz.com
[ANSWERED] The energy of a hydrogen atom in the ground state is 13 6 eV Bromine Atom In The Ground State From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. That is, we follow the three important rules:. Total number of protons in the nucleus is called the atomic number of the. Bromine Atom In The Ground State.
From exonchlhy.blob.core.windows.net
What Is The Complete Ground State Electron Configuration For The Bromine Atom In The Ground State That is, we follow the three important rules:. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. From the electrons in. Bromine Atom In The Ground State.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Add an electron to the anion. If an atom has lost or gained electrons by changing to a cation or anion, follow these. Bromine Atom In The Ground State.
From ar.inspiredpencil.com
Potassium Model Bromine Atom In The Ground State The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. That is, we follow the three important rules:. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Ground state electron configurations are the. Bromine Atom In The Ground State.
From www.carnews.com.mx
カテゴリ∊ BRの通販 セイゴ 's shop|ラクマ by カテゴリ Bromine Atom In The Ground State The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. From the electrons in an atom, to the differing orbitals and hybridization,. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Fundamentally, understanding electron configuration leads to an understanding of the periodic. Bromine Atom In The Ground State.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Bromine Atom In The Ground State From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms. Bromine Atom In The Ground State.
From chem.libretexts.org
8.7 SpinOrbitals and Electron Configurations Chemistry LibreTexts Bromine Atom In The Ground State The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Total number of protons. Bromine Atom In The Ground State.
From kizakingdom.weebly.com
Ground state electron configuration kizakingdom Bromine Atom In The Ground State Add an electron to the anion. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p. Bromine Atom In The Ground State.
From games.assurances.gov.gh
Br Electron Configuration Long Form Bromine Atom In The Ground State Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to. Bromine Atom In The Ground State.
From www.vrogue.co
How To Write Ground State Electron Configuration For vrogue.co Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Fundamentally, understanding electron configuration leads to an understanding of the periodic table. Add an electron to the anion. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. The ground state electron configuration of ground. Bromine Atom In The Ground State.
From www.youtube.com
Two H atoms in the ground state collide inelastically. The maximum Bromine Atom In The Ground State Add an electron to the anion. From the electrons in an atom, to the differing orbitals and hybridization, the ground state electron configuration sheds light on many different atomic properties. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. The. Bromine Atom In The Ground State.
From www.numerade.com
SOLVED Use the uncertainty principle to estimate A) the ground state Bromine Atom In The Ground State Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Fundamentally, understanding electron configuration leads to an understanding of. Bromine Atom In The Ground State.
From askfilo.com
A collection of Hatoms in 9th excited state returns to ground state. C.. Bromine Atom In The Ground State The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus. Add an electron to the anion. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s. Bromine Atom In The Ground State.
From www.jahaneshimi.com
عنصر کریپتون چیست؟ درباره کاربرد کریپتون چه می دانید؟ جهان شیمی فیزیک Bromine Atom In The Ground State Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Add an electron to the anion. If an atom has lost or gained electrons by changing to a cation or anion, follow these steps: That is, we follow the three important rules:. Bromine is a. Bromine Atom In The Ground State.