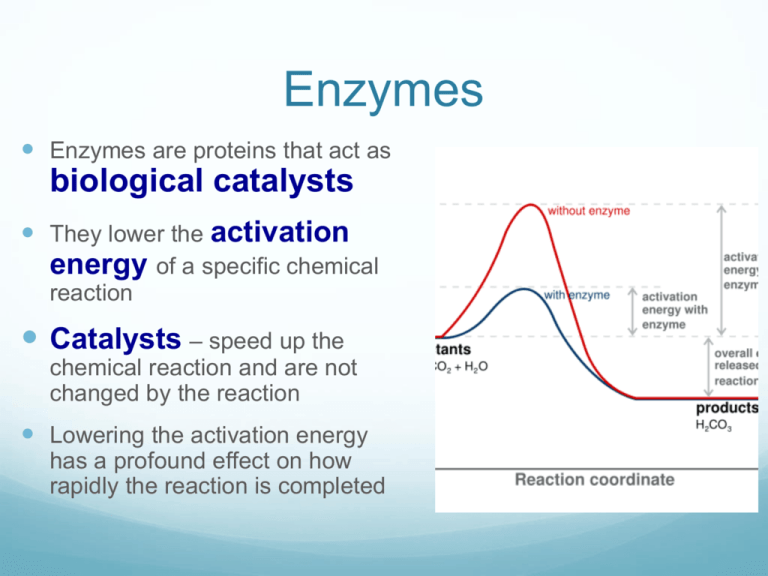
Catalyst Lower Activation Energy Of A Reaction . It does not lower the activation energy of the reaction. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. There is a subtle difference between the two statements that is easily illustrated with a Adding a catalyst has exactly this effect of shifting the activation energy. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. A catalyst binds to a reactant and it increases the number of collision between. A catalyst provides an alternative route for the reaction with a lower activation energy. That alternative route has a lower activation energy. A catalyst provides an alternative route for the reaction. In heterogeneous catalysis, catalysts provide a surface to.
from studylib.net
A catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. A catalyst provides an alternative route for the reaction. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. That alternative route has a lower activation energy. In heterogeneous catalysis, catalysts provide a surface to. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. There is a subtle difference between the two statements that is easily illustrated with a Adding a catalyst has exactly this effect of shifting the activation energy.
Enzymes Lower Activation Energy
Catalyst Lower Activation Energy Of A Reaction Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. A catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. Adding a catalyst has exactly this effect of shifting the activation energy. There is a subtle difference between the two statements that is easily illustrated with a It does not lower the activation energy of the reaction. A catalyst binds to a reactant and it increases the number of collision between. That alternative route has a lower activation energy. A catalyst provides an alternative route for the reaction. In heterogeneous catalysis, catalysts provide a surface to.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Catalyst Lower Activation Energy Of A Reaction In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. In heterogeneous catalysis, catalysts provide a surface to. Adding a catalyst has exactly this effect of shifting the activation. Catalyst Lower Activation Energy Of A Reaction.
From professional.patrickneyman.com
Activation Energy Equation Catalyst Lower Activation Energy Of A Reaction In heterogeneous catalysis, catalysts provide a surface to. A catalyst provides an alternative route for the reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. That alternative route has a lower activation energy. The more dramatic effect of the catalyst involves the provision of an. Catalyst Lower Activation Energy Of A Reaction.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Lower Activation Energy Of A Reaction In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. In heterogeneous catalysis, catalysts provide a surface to. It does not lower the activation energy of the reaction. A. Catalyst Lower Activation Energy Of A Reaction.
From www.chim.lu
Activation energy and catalysis Catalyst Lower Activation Energy Of A Reaction When a reaction has a lower activation energy, it occurs more readily and thus more quickly. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. In heterogeneous catalysis, catalysts provide a surface to. There is a subtle difference between the two statements that is easily illustrated. Catalyst Lower Activation Energy Of A Reaction.
From www.slideserve.com
PPT Reaction PowerPoint Presentation ID1835084 Catalyst Lower Activation Energy Of A Reaction It does not lower the activation energy of the reaction. A catalyst binds to a reactant and it increases the number of collision between. Adding a catalyst has exactly this effect of shifting the activation energy. That alternative route has a lower activation energy. When a reaction has a lower activation energy, it occurs more readily and thus more quickly.. Catalyst Lower Activation Energy Of A Reaction.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Lower Activation Energy Of A Reaction Adding a catalyst has exactly this effect of shifting the activation energy. That alternative route has a lower activation energy. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. There is a subtle difference between the two statements that is easily illustrated with a A catalyst provides an alternative route for the reaction.. Catalyst Lower Activation Energy Of A Reaction.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.2.4 Activation Energy翰林国际教育 Catalyst Lower Activation Energy Of A Reaction That alternative route has a lower activation energy. A catalyst binds to a reactant and it increases the number of collision between. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. Adding a catalyst has exactly this effect of shifting the activation energy. It does not lower the activation energy of. Catalyst Lower Activation Energy Of A Reaction.
From byjus.com
Activation Energy Catalyst Lower Activation Energy Of A Reaction In heterogeneous catalysis, catalysts provide a surface to. That alternative route has a lower activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. There is a subtle difference between the two statements that is easily illustrated with a Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides. Catalyst Lower Activation Energy Of A Reaction.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Lower Activation Energy Of A Reaction That alternative route has a lower activation energy. A catalyst binds to a reactant and it increases the number of collision between. A catalyst provides an alternative route for the reaction with a lower activation energy. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. The more dramatic effect of the catalyst involves. Catalyst Lower Activation Energy Of A Reaction.
From ar.inspiredpencil.com
Enzymes Activation Energy Catalyst Lower Activation Energy Of A Reaction When a reaction has a lower activation energy, it occurs more readily and thus more quickly. In heterogeneous catalysis, catalysts provide a surface to. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the reaction. In the case of a biological reaction, when an enzyme (a form of. Catalyst Lower Activation Energy Of A Reaction.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz Catalyst Lower Activation Energy Of A Reaction Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the reaction. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. That alternative route has a lower activation energy. There is a subtle difference between the two statements that is easily illustrated with a. Catalyst Lower Activation Energy Of A Reaction.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Catalyst Lower Activation Energy Of A Reaction Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction. A catalyst binds to a reactant and it increases the number of collision between. That alternative route. Catalyst Lower Activation Energy Of A Reaction.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Lower Activation Energy Of A Reaction A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the reaction. In heterogeneous catalysis, catalysts provide a surface to. It does not lower the activation energy of the reaction. There is a subtle difference between the two statements that is easily illustrated with a When a reaction has. Catalyst Lower Activation Energy Of A Reaction.
From www.energyfrontier.us
When Less Is More Energy Frontier Research Center Catalyst Lower Activation Energy Of A Reaction A catalyst binds to a reactant and it increases the number of collision between. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Adding a catalyst has exactly this effect of shifting the activation energy. In heterogeneous catalysis, catalysts provide a surface to. That alternative route. Catalyst Lower Activation Energy Of A Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Lower Activation Energy Of A Reaction Adding a catalyst has exactly this effect of shifting the activation energy. In heterogeneous catalysis, catalysts provide a surface to. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. There is a subtle difference between the two statements that is easily illustrated with a In the case of a biological reaction, when an. Catalyst Lower Activation Energy Of A Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Lower Activation Energy Of A Reaction A catalyst binds to a reactant and it increases the number of collision between. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts. Catalyst Lower Activation Energy Of A Reaction.
From www.sliderbase.com
Catalysis Presentation Chemistry Catalyst Lower Activation Energy Of A Reaction There is a subtle difference between the two statements that is easily illustrated with a A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. Adding a catalyst has exactly this effect of shifting the. Catalyst Lower Activation Energy Of A Reaction.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Lower Activation Energy Of A Reaction It does not lower the activation energy of the reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst binds to a reactant and it increases the number of collision between. There is a subtle difference between the two statements that is easily illustrated with a In heterogeneous catalysis, catalysts provide a surface. Catalyst Lower Activation Energy Of A Reaction.
From fixportillobadgered.z21.web.core.windows.net
Energy Diagram Activation Energy Catalyst Lower Activation Energy Of A Reaction That alternative route has a lower activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. A catalyst binds to a reactant and it increases the number of collision between. It does not lower the activation energy of. Catalyst Lower Activation Energy Of A Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Lower Activation Energy Of A Reaction When a reaction has a lower activation energy, it occurs more readily and thus more quickly. A catalyst binds to a reactant and it increases the number of collision between. There is a subtle difference between the two statements that is easily illustrated with a It does not lower the activation energy of the reaction. In the case of a. Catalyst Lower Activation Energy Of A Reaction.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalyst Lower Activation Energy Of A Reaction It does not lower the activation energy of the reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. Adding a catalyst has exactly this effect of shifting the activation energy. In heterogeneous catalysis, catalysts provide a surface to. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to. Catalyst Lower Activation Energy Of A Reaction.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Catalyst Lower Activation Energy Of A Reaction Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. In heterogeneous catalysis, catalysts provide a surface to. It does not lower the activation energy of the reaction. That alternative route has a lower activation. Catalyst Lower Activation Energy Of A Reaction.
From wisc.pb.unizin.org
Day 24 Multistep Reaction Mechanisms Chemistry 109 Catalyst Lower Activation Energy Of A Reaction A catalyst binds to a reactant and it increases the number of collision between. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In the case of. Catalyst Lower Activation Energy Of A Reaction.
From 2012books.lardbucket.org
Catalysis Catalyst Lower Activation Energy Of A Reaction Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Adding a catalyst has exactly this effect of shifting the activation energy. In heterogeneous catalysis, catalysts provide a surface to. A catalyst binds to a reactant and it increases the number of collision between. It does not lower the activation energy. Catalyst Lower Activation Energy Of A Reaction.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Lower Activation Energy Of A Reaction A catalyst provides an alternative route for the reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction. Adding a catalyst has exactly this effect of shifting the activation energy. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy. Catalyst Lower Activation Energy Of A Reaction.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Lower Activation Energy Of A Reaction The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. In heterogeneous catalysis, catalysts provide a surface to. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. There is a subtle difference between the two statements that is easily illustrated with a Catalysts allow a. Catalyst Lower Activation Energy Of A Reaction.
From thebiologs.blogspot.com
The BioLogs Ezymes CSEC Catalyst Lower Activation Energy Of A Reaction When a reaction has a lower activation energy, it occurs more readily and thus more quickly. A catalyst provides an alternative route for the reaction. Adding a catalyst has exactly this effect of shifting the activation energy. There is a subtle difference between the two statements that is easily illustrated with a A catalyst provides an alternative route for the. Catalyst Lower Activation Energy Of A Reaction.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Lower Activation Energy Of A Reaction A catalyst provides an alternative route for the reaction with a lower activation energy. That alternative route has a lower activation energy. It does not lower the activation energy of the reaction. Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the reaction. There is a subtle difference between the. Catalyst Lower Activation Energy Of A Reaction.
From socratic.org
What are activation energies? Socratic Catalyst Lower Activation Energy Of A Reaction It does not lower the activation energy of the reaction. There is a subtle difference between the two statements that is easily illustrated with a In heterogeneous catalysis, catalysts provide a surface to. A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy. The more dramatic effect of the catalyst involves the provision. Catalyst Lower Activation Energy Of A Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Lower Activation Energy Of A Reaction It does not lower the activation energy of the reaction. Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst binds to a reactant and it increases the number of collision between. That alternative route has a lower activation energy. A catalyst provides an alternative route for the reaction. In the case of a biological reaction,. Catalyst Lower Activation Energy Of A Reaction.
From studylib.net
Enzymes Lower Activation Energy Catalyst Lower Activation Energy Of A Reaction That alternative route has a lower activation energy. Adding a catalyst has exactly this effect of shifting the activation energy. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. There is a subtle difference between the two statements that is easily illustrated with a A catalyst provides an alternative route for. Catalyst Lower Activation Energy Of A Reaction.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Catalyst Lower Activation Energy Of A Reaction A catalyst binds to a reactant and it increases the number of collision between. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. There is. Catalyst Lower Activation Energy Of A Reaction.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Lower Activation Energy Of A Reaction In heterogeneous catalysis, catalysts provide a surface to. It does not lower the activation energy of the reaction. A catalyst provides an alternative route for the reaction. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism with lower activation. There is a subtle difference between the two statements that is easily illustrated with a. Catalyst Lower Activation Energy Of A Reaction.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyst Lower Activation Energy Of A Reaction When a reaction has a lower activation energy, it occurs more readily and thus more quickly. There is a subtle difference between the two statements that is easily illustrated with a A catalyst binds to a reactant and it increases the number of collision between. The more dramatic effect of the catalyst involves the provision of an alternative reaction mechanism. Catalyst Lower Activation Energy Of A Reaction.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Lower Activation Energy Of A Reaction A catalyst binds to a reactant and it increases the number of collision between. When a reaction has a lower activation energy, it occurs more readily and thus more quickly. That alternative route has a lower activation energy. In heterogeneous catalysis, catalysts provide a surface to. Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst. Catalyst Lower Activation Energy Of A Reaction.