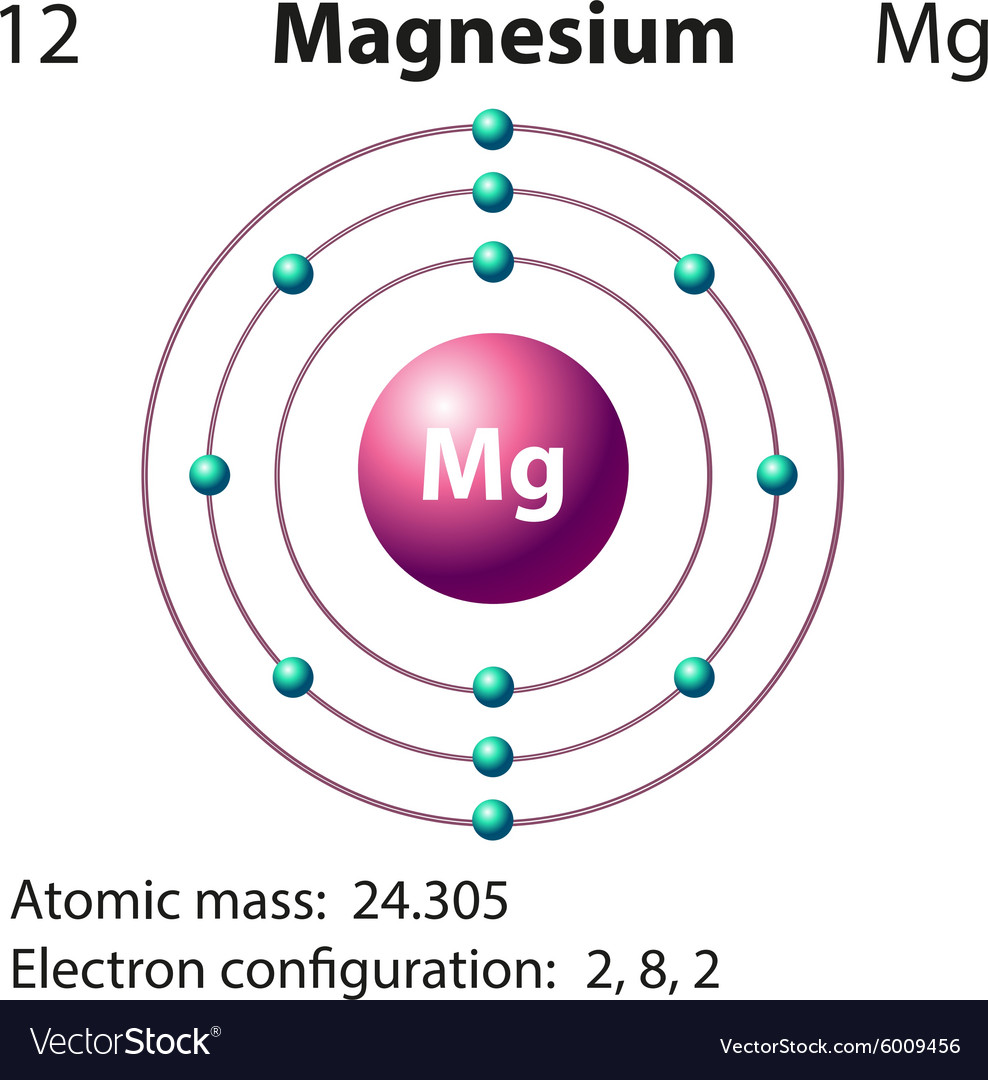
Magnesium Electron Orbital Diagram . This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where. Magnesium has two valence electrons in the 3s orbital. Today we will tell you about the electron configuration of the mg. The ninth most abundant element in the universe. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Since the 1s orbital can hold only two electrons the next two.
from www.vectorstock.com
All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Magnesium has two valence electrons in the 3s orbital. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Since the 1s orbital can hold only two electrons the next two. The ninth most abundant element in the universe. Today we will tell you about the electron configuration of the mg. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Orbital is the region of space around the nucleus of an atom where.
Diagram representation of the element magnesium Vector Image
Magnesium Electron Orbital Diagram Today we will tell you about the electron configuration of the mg. Since the 1s orbital can hold only two electrons the next two. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. The ninth most abundant element in the universe. Today we will tell you about the electron configuration of the mg. Orbital is the region of space around the nucleus of an atom where. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Electron Orbital Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Today we will tell you about the electron configuration of the mg. Magnesium has two valence electrons in the 3s orbital. Orbital is the region of space around the nucleus of an atom where. To. Magnesium Electron Orbital Diagram.
From learningdbwakefully.z5.web.core.windows.net
Electron Configurations And Orbital Diagrams Worksheets Magnesium Electron Orbital Diagram To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Since the 1s orbital can hold only two electrons the next two. Today we will tell you about the electron configuration of the mg. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. The ninth most abundant. Magnesium Electron Orbital Diagram.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Electron Orbital Diagram Since the 1s orbital can hold only two electrons the next two. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To illustrate the. Magnesium Electron Orbital Diagram.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Electron Orbital Diagram This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Today we will tell you about the electron configuration of the mg. Since the 1s orbital can hold only two electrons the next two. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Orbital is the region. Magnesium Electron Orbital Diagram.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Magnesium Electron Orbital Diagram The ninth most abundant element in the universe. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. To illustrate the magnesium orbital diagram, start by determining the number of electrons from. Magnesium Electron Orbital Diagram.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Electron Orbital Diagram Orbital is the region of space around the nucleus of an atom where. Since the 1s orbital can hold only two electrons the next two. Today we will tell you about the electron configuration of the mg. The ninth most abundant element in the universe. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals.. Magnesium Electron Orbital Diagram.
From mavink.com
Electron Orbital Configuration Chart Magnesium Electron Orbital Diagram The ninth most abundant element in the universe. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Orbital is the region of space around the nucleus of an atom where. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron. Magnesium Electron Orbital Diagram.
From www.meritnation.com
I want a labelled diagram of the atom of magnesium not the atomic no or Magnesium Electron Orbital Diagram Orbital is the region of space around the nucleus of an atom where. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Today we will tell you about the electron configuration of the mg. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. To write the electron configuration for magnesium, the. Magnesium Electron Orbital Diagram.
From ar.inspiredpencil.com
Magnesium Orbital Diagram Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where. Today we will tell you about the electron configuration of the mg. Since the 1s orbital can hold only two electrons the next. Magnesium Electron Orbital Diagram.
From chemistrytalk.org
Orbital Diagrams ChemTalk Magnesium Electron Orbital Diagram Since the 1s orbital can hold only two electrons the next two. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. The ninth most abundant element in the universe. Today we will tell you about the electron configuration of the mg. To write the electron configuration for magnesium, the first two electrons enter the. Magnesium Electron Orbital Diagram.
From valeriadoyle.blogspot.com
orbital diagram for magnesium ValeriaDoyle Magnesium Electron Orbital Diagram This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where. Since the 1s orbital can hold only two electrons the next two. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To illustrate the magnesium orbital diagram, start by determining the. Magnesium Electron Orbital Diagram.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Electron Orbital Diagram Since the 1s orbital can hold only two electrons the next two. Magnesium has two valence electrons in the 3s orbital. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Orbital. Magnesium Electron Orbital Diagram.
From ar.inspiredpencil.com
Magnesium Orbital Diagram Magnesium Electron Orbital Diagram This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. The ninth most abundant element in the universe. Magnesium has two valence electrons in the 3s orbital. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Today we will tell you about the electron configuration of the mg. Since. Magnesium Electron Orbital Diagram.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. The ninth most abundant element in the universe. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Today we will tell you about the electron configuration of the mg. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic. Magnesium Electron Orbital Diagram.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Magnesium Electron Orbital Diagram To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Today we will tell you about the electron configuration of the mg. Magnesium has two valence electrons in the 3s orbital. To illustrate the magnesium orbital diagram, start by determining. Magnesium Electron Orbital Diagram.
From ar.inspiredpencil.com
Magnesium Orbital Diagram Magnesium Electron Orbital Diagram The ninth most abundant element in the universe. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Today we will tell you about the electron configuration of the mg. The electron configuration of magnesium is 1s² 2s². Magnesium Electron Orbital Diagram.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Electron Orbital Diagram To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Orbital is the region of space around the nucleus of. Magnesium Electron Orbital Diagram.
From uzabytaodi.blogspot.com
41 orbital diagram of magnesium Wiring Diagrams Manual Magnesium Electron Orbital Diagram The ninth most abundant element in the universe. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Since the 1s orbital can hold only two electrons the next two. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. The electron configuration of magnesium is 1s² 2s². Magnesium Electron Orbital Diagram.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Orbital Diagram Since the 1s orbital can hold only two electrons the next two. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. The ninth most abundant element in the universe. Orbital is the region of space around the nucleus of an atom where. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To illustrate. Magnesium Electron Orbital Diagram.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Orbital Diagram Since the 1s orbital can hold only two electrons the next two. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where. The ninth most abundant element in the universe. Magnesium has two valence electrons in the 3s orbital. The electron configuration. Magnesium Electron Orbital Diagram.
From www.toppr.com
Give orbital diagram of the followingmagnesium chloride, Magnesium Electron Orbital Diagram To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Magnesium has two valence electrons in the 3s orbital. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. All elements of group. Magnesium Electron Orbital Diagram.
From manuallistcantabank.z21.web.core.windows.net
Orbital Filling Diagram Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. Orbital is the region of space around the nucleus of an atom where. The ninth most abundant element in the universe. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. All elements of group 2 have the same configuration of an electron in the outer. Magnesium Electron Orbital Diagram.
From wiringfixunripping.z21.web.core.windows.net
Valence Electrons Orbital Diagram Magnesium Electron Orbital Diagram To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Magnesium Electron Orbital Diagram.
From chicfer.blogspot.com
orbital diagram magnesium Chicfer Magnesium Electron Orbital Diagram This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Since the 1s orbital can hold only two electrons the next two. Magnesium has two valence electrons in the 3s orbital. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron. Magnesium Electron Orbital Diagram.
From valeriadoyle.blogspot.com
orbital diagram for magnesium ValeriaDoyle Magnesium Electron Orbital Diagram The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Since the 1s orbital can hold only two electrons the next two. Today we will tell you about the electron configuration of the mg. The ninth. Magnesium Electron Orbital Diagram.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Orbital is the region of space around the nucleus of an atom where. All elements of group 2 have. Magnesium Electron Orbital Diagram.
From mungfali.com
Magnesium Orbital Diagram Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Orbital is the region of space around the nucleus of an atom where. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure.. Magnesium Electron Orbital Diagram.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Today we will tell you about the electron configuration of the mg. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Since the 1s orbital can hold only. Magnesium Electron Orbital Diagram.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Orbital Diagram To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Since the 1s orbital can hold only two electrons the next two. To write the electron configuration for magnesium, the. Magnesium Electron Orbital Diagram.
From www.clipartkey.com
Electrons Ck Foundation Look Orbital Energy Diagram Magnesium , Free Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where. All elements of group 2 have the same configuration of an electron in. Magnesium Electron Orbital Diagram.
From quicycle.com
12. Magnesium The Quantum Bicycle Society Magnesium Electron Orbital Diagram This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Magnesium Electron Orbital Diagram.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. Orbital is the region of space around the nucleus of an atom where. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. All elements of group 2 have the same configuration of an electron in. Magnesium Electron Orbital Diagram.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Magnesium Electron Orbital Diagram To write the electron configuration for magnesium, the first two electrons enter the 1s orbital. Orbital is the region of space around the nucleus of an atom where. Magnesium has two valence electrons in the 3s orbital. Today we will tell you about the electron configuration of the mg. This diagram shows how the electrons in the magnesium atom are. Magnesium Electron Orbital Diagram.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Magnesium Electron Orbital Diagram Orbital is the region of space around the nucleus of an atom where. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. This diagram shows how the. Magnesium Electron Orbital Diagram.
From www.slideserve.com
PPT Orbital Diagrams PowerPoint Presentation ID6677860 Magnesium Electron Orbital Diagram Magnesium has two valence electrons in the 3s orbital. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Orbital is the region of space around the nucleus of an atom where. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the. Magnesium Electron Orbital Diagram.