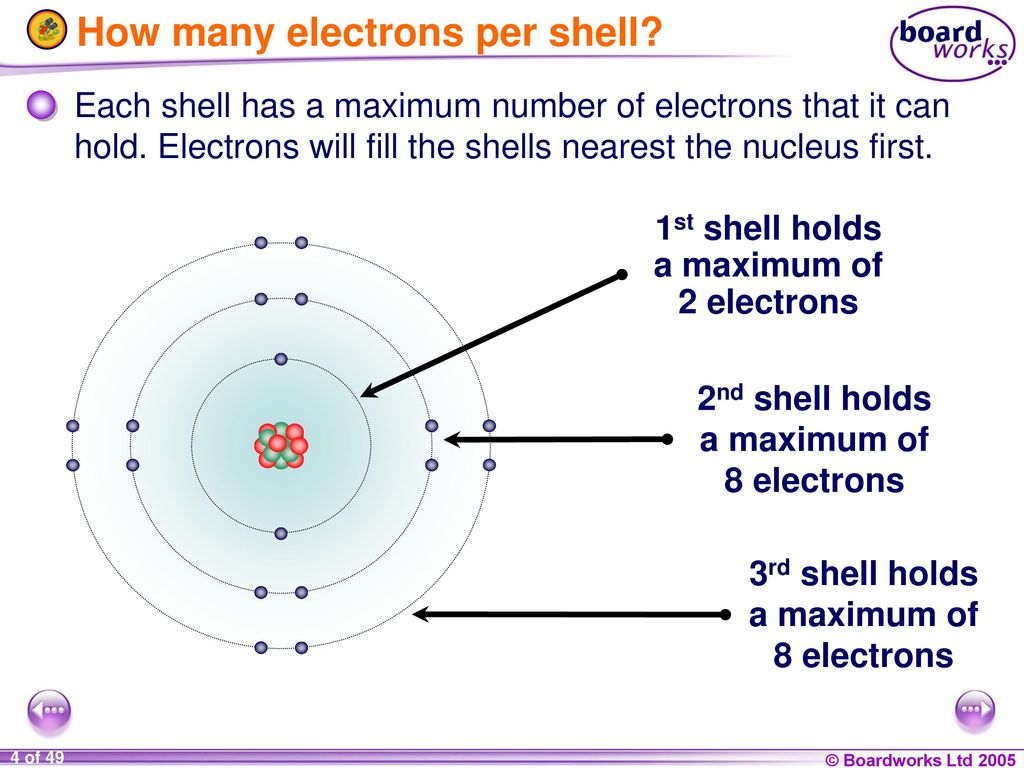
Copper Number Of Electrons Per Shell . It is located in group. The innermost shell has a. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. Sources, facts, uses, scarcity (sri), podcasts, alchemical.
from slideplayer.com
under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. It is located in group. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The innermost shell has a. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. In other words, it's the sum. The sum of the number of protons and neutrons of an atomic nucleus. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling.
Electronic structure and the periodic table ppt download
Copper Number Of Electrons Per Shell The innermost shell has a. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. In other words, it's the sum. Sources, facts, uses, scarcity (sri), podcasts, alchemical. It is located in group. The sum of the number of protons and neutrons of an atomic nucleus. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. The innermost shell has a.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Copper (Cu) YouTube Copper Number Of Electrons Per Shell Sources, facts, uses, scarcity (sri), podcasts, alchemical. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. In other words, it's the sum. The innermost shell has a. The sum of the number of protons and neutrons of an atomic nucleus. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰,. Copper Number Of Electrons Per Shell.
From www.wou.edu
Ch105 Chapter 2 Atoms, Elements and The Periodic Table Chemistry Copper Number Of Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. Sources, facts, uses, scarcity (sri), podcasts, alchemical. It is located in group. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The sum. Copper Number Of Electrons Per Shell.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper Number Of Electrons Per Shell Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. In other words, it's the sum. The sum of the number of protons and neutrons of an atomic nucleus. how to write the electron configuration for. Copper Number Of Electrons Per Shell.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Number Of Electrons Per Shell In other words, it's the sum. It is located in group. The innermost shell has a. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The sum of the number of protons and neutrons of an atomic nucleus. how to write the electron configuration for copper. Copper Number Of Electrons Per Shell.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Number Of Electrons Per Shell Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum. It is located in group. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The innermost shell has a. 119 rows the list below gives the elements arranged by increasing atomic. Copper Number Of Electrons Per Shell.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Copper Number Of Electrons Per Shell In other words, it's the sum. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. The innermost shell has a. The sum of the number of protons and neutrons of an atomic nucleus. Sources, facts, uses, scarcity (sri), podcasts, alchemical. It is located in group.. Copper Number Of Electrons Per Shell.
From sciencenotes.org
Periodic Table Showing Shells Copper Number Of Electrons Per Shell Sources, facts, uses, scarcity (sri), podcasts, alchemical. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. It is located in group. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. In other words, it's the sum. 119 rows the list below gives. Copper Number Of Electrons Per Shell.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name, atomic weight and electrons Copper Number Of Electrons Per Shell It is located in group. The sum of the number of protons and neutrons of an atomic nucleus. The innermost shell has a. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. under standard conditions, atoms fill the inner shells first, often resulting in. Copper Number Of Electrons Per Shell.
From stock.adobe.com
Periodic Table of the Elements, Shell Structure of Copper Cu Electrons per energy level. Stock Copper Number Of Electrons Per Shell under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. In other words, it's the sum. The sum of the number of protons and neutrons of an atomic nucleus. It is located in group. 119 rows the list below gives the elements arranged by increasing atomic number. Copper Number Of Electrons Per Shell.
From ar.inspiredpencil.com
Periodic Table Electron Shells Copper Number Of Electrons Per Shell Sources, facts, uses, scarcity (sri), podcasts, alchemical. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order. Copper Number Of Electrons Per Shell.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Number Of Electrons Per Shell In other words, it's the sum. The sum of the number of protons and neutrons of an atomic nucleus. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in. Copper Number Of Electrons Per Shell.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper Number Of Electrons Per Shell under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The sum of the number of protons and neutrons of an atomic nucleus. It is located in group. Sources, facts, uses, scarcity (sri), podcasts, alchemical. 119 rows the list below gives the elements arranged by increasing atomic. Copper Number Of Electrons Per Shell.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Copper Number Of Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The sum of the number of protons and neutrons of an atomic nucleus. Sources,. Copper Number Of Electrons Per Shell.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol, name, atomic weight Copper Number Of Electrons Per Shell The sum of the number of protons and neutrons of an atomic nucleus. The innermost shell has a. In other words, it's the sum. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. 119 rows the list below gives the elements arranged by increasing atomic number. Copper Number Of Electrons Per Shell.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper Number Of Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. It is located in group. Sources, facts, uses, scarcity (sri), podcasts, alchemical. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. The sum. Copper Number Of Electrons Per Shell.
From slideplayer.com
Electronic structure and the periodic table ppt download Copper Number Of Electrons Per Shell 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. In other words, it's the sum. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. It is located in group. The sum of. Copper Number Of Electrons Per Shell.
From www.britannica.com
Electron shell Definition & Facts Britannica Copper Number Of Electrons Per Shell It is located in group. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The innermost shell has a. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons. Copper Number Of Electrons Per Shell.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name, atomic weight, electrons per Copper Number Of Electrons Per Shell The innermost shell has a. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. In other words, it's the sum. It is located in group. Sources, facts, uses, scarcity (sri), podcasts, alchemical. how to write. Copper Number Of Electrons Per Shell.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Number Of Electrons Per Shell In other words, it's the sum. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. The innermost shell has a. Sources, facts, uses, scarcity (sri), podcasts, alchemical. It is located in group. 119. Copper Number Of Electrons Per Shell.
From www.slideshare.net
Copper Copper Number Of Electrons Per Shell It is located in group. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. Sources, facts, uses, scarcity (sri), podcasts, alchemical. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. The innermost. Copper Number Of Electrons Per Shell.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Copper Number Of Electrons Per Shell The innermost shell has a. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. It is located in group. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write. Copper Number Of Electrons Per Shell.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Number Of Electrons Per Shell In other words, it's the sum. Sources, facts, uses, scarcity (sri), podcasts, alchemical. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a. The sum of the number of protons and neutrons of an atomic nucleus. It is located in group. 119. Copper Number Of Electrons Per Shell.
From en-academic.com
Copper Copper Number Of Electrons Per Shell Sources, facts, uses, scarcity (sri), podcasts, alchemical. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. In other words, it's the sum. The innermost shell has a. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. under standard conditions, atoms fill the inner shells. Copper Number Of Electrons Per Shell.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name, atomic weight, electrons per Copper Number Of Electrons Per Shell 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. The innermost shell has a. In other words, it's the sum. Sources, facts, uses, scarcity (sri), podcasts, alchemical. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the. Copper Number Of Electrons Per Shell.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Number Of Electrons Per Shell Sources, facts, uses, scarcity (sri), podcasts, alchemical. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. In other words, it's. Copper Number Of Electrons Per Shell.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Number Of Electrons Per Shell Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The innermost shell has a. In other words, it's the sum. It is located in group. The sum of the number of protons and neutrons of an atomic nucleus. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the. Copper Number Of Electrons Per Shell.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Number Of Electrons Per Shell under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. It is located in group. The sum of the number of protons and neutrons of an atomic nucleus. Sources, facts, uses, scarcity (sri), podcasts, alchemical. 119 rows the list below gives the elements arranged by increasing atomic. Copper Number Of Electrons Per Shell.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Copper vs Aluminium Compare Properties, Structure Copper Number Of Electrons Per Shell It is located in group. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. In other words, it's the sum. The innermost shell has a. Sources, facts, uses, scarcity (sri), podcasts, alchemical. under standard conditions, atoms fill the inner shells first, often resulting in a variable. Copper Number Of Electrons Per Shell.
From cronodon.com
Introduction to Atoms Copper Number Of Electrons Per Shell In other words, it's the sum. The sum of the number of protons and neutrons of an atomic nucleus. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron. Copper Number Of Electrons Per Shell.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol, name, atomic weight Copper Number Of Electrons Per Shell It is located in group. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The innermost shell has a. In other words, it's the sum. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The sum of the number of protons and neutrons of an atomic nucleus. Copper’s electron. Copper Number Of Electrons Per Shell.
From guidelibsolidarity.z21.web.core.windows.net
Periodic Table With Electron Dot Diagrams Copper Number Of Electrons Per Shell The innermost shell has a. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The sum of the number of protons and neutrons of an atomic nucleus. It is located in group. how to write. Copper Number Of Electrons Per Shell.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Number Of Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. In other words, it's the sum. The innermost shell has a. The sum of the number of protons and neutrons of an atomic nucleus. under standard conditions, atoms fill the inner shells first, often resulting. Copper Number Of Electrons Per Shell.
From utedzz.blogspot.com
Fully Labeled Periodic Table With Names Periodic Table Timeline Copper Number Of Electrons Per Shell under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The sum of the number of protons and neutrons of an atomic nucleus. The innermost shell has a. It is located in group. how to write the electron configuration for copper (cu, cu+, and cu2+) in order. Copper Number Of Electrons Per Shell.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper Number Of Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. It is located in group. Sources, facts, uses, scarcity (sri), podcasts, alchemical. The innermost shell has a. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of. Copper Number Of Electrons Per Shell.
From schematicdatavenin77.z5.web.core.windows.net
Periodic Table With Electron Dot Diagrams Copper Number Of Electrons Per Shell The sum of the number of protons and neutrons of an atomic nucleus. under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. 119 rows the list below gives the elements arranged by increasing atomic number and shows the number of electrons per shell. The innermost shell. Copper Number Of Electrons Per Shell.