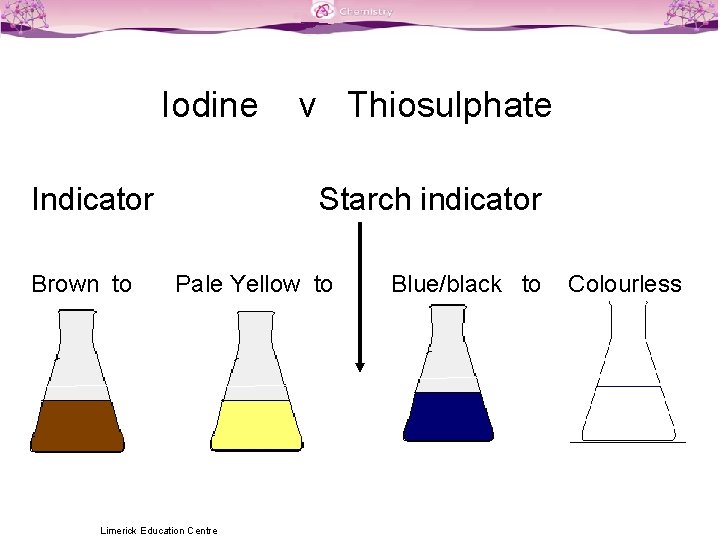
Why Is Sodium Thiosulfate Used In Iodometric Titration . this titration process will use sodium thiosulfate (na2s2o3). Then it is titrated with sodium thiosulfate 0.1 mol/l to an. solutions used in iodometric titrations. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. 1.5 g of ki are added. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Two most important solutions used in iodometric titrations are solution.
from slidetodoc.com
Two most important solutions used in iodometric titrations are solution. solutions used in iodometric titrations. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. this titration process will use sodium thiosulfate (na2s2o3). 1.5 g of ki are added.
Titration Colour Changes SLSS Science Limerick Education Centre
Why Is Sodium Thiosulfate Used In Iodometric Titration Two most important solutions used in iodometric titrations are solution. solutions used in iodometric titrations. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. 1.5 g of ki are added. Two most important solutions used in iodometric titrations are solution. this titration process will use sodium thiosulfate (na2s2o3). arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine.
From ceyuitwn.blob.core.windows.net
Indicator Titration Purpose at Richard Neumann blog Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. this titration process will use sodium thiosulfate (na2s2o3). a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3),. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.youtube.com
Eureka 2017 Iodometry Redox Titration KIO3 Vs Thiosulfate YouTube Why Is Sodium Thiosulfate Used In Iodometric Titration Then it is titrated with sodium thiosulfate 0.1 mol/l to an. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Two most important solutions used in iodometric titrations are solution. solutions used in iodometric titrations. this titration process will use sodium thiosulfate (na2s2o3). 1.5. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From dmkpehdeeco.blob.core.windows.net
Indicator For Neutralization Titration at William Wiesner blog Why Is Sodium Thiosulfate Used In Iodometric Titration solutions used in iodometric titrations. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. 1.5 g of ki are added. Two most important solutions used in iodometric titrations are solution. this titration process. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From byjus.com
Na thiosulfate in iodine titration methods Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). Then it is titrated with sodium thiosulfate 0.1 mol/l to an. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. 1.5 g of ki are added. Two most important solutions used in iodometric titrations are solution. arsenic oxide is dissolved in sodium hydroxide, producing. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Why Is Sodium Thiosulfate Used In Iodometric Titration Then it is titrated with sodium thiosulfate 0.1 mol/l to an. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. this titration process will use sodium thiosulfate (na2s2o3). arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. a precise and. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration Then it is titrated with sodium thiosulfate 0.1 mol/l to an. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. solutions used in iodometric titrations. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Two most. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). Two most important solutions used in iodometric titrations are solution. solutions used in iodometric titrations. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. 1.5 g of. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). This sodium thiosulfate is also known as a reducing agent to titrate the iodine. solutions used in iodometric titrations. 1.5 g of ki are added. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. arsenic oxide is dissolved. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. this titration process will use sodium thiosulfate (na2s2o3). Two most important solutions used in iodometric titrations are solution. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. . Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Two most important solutions used in iodometric titrations are solution. this titration process will use sodium thiosulfate (na2s2o3). solutions used in iodometric titrations. 1.5 g of ki are added. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. arsenic oxide. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From study.com
Sodium Thiosulfate & Hydrochloric Acid Experiment Lesson Why Is Sodium Thiosulfate Used In Iodometric Titration a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. this titration process will use sodium thiosulfate (na2s2o3). Two most important solutions used in iodometric titrations are solution. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. iodine. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration solutions used in iodometric titrations. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. this titration process will use sodium thiosulfate (na2s2o3). iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. this titration process will use sodium thiosulfate (na2s2o3). This sodium thiosulfate is also known as a reducing agent to titrate the iodine. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.youtube.com
Iodine and sodium thiosulfate redox titration calculations ALevel Chemistry YouTube Why Is Sodium Thiosulfate Used In Iodometric Titration iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. 1.5 g of ki are added. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. solutions used in iodometric titrations. Then it is titrated with sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From mavink.com
Sodium Thiosulfate Titration Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Two most important solutions used in iodometric titrations are. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From quizspattering.z21.web.core.windows.net
How To Find Equivalence Point Of Titration Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. 1.5 g of ki are added. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite,. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Two most important solutions used in iodometric titrations are solution. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.youtube.com
Sodium Thiosulphate Solution preparation and Standardization Iodometric titration Concept Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). 1.5 g of ki are added. solutions used in iodometric titrations. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Then it is titrated with. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. this titration process will use sodium thiosulfate (na2s2o3). solutions used in iodometric titrations. a precise and stable reducing agent, sodium thiosulfate. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration TutorMyself Chemistry Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. solutions used in iodometric titrations. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Two most important solutions used in iodometric titrations are solution. . Why Is Sodium Thiosulfate Used In Iodometric Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Why Is Sodium Thiosulfate Used In Iodometric Titration This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Two most important solutions used in iodometric titrations are solution. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. a precise and stable reducing agent, sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Why Is Sodium Thiosulfate Used In Iodometric Titration Then it is titrated with sodium thiosulfate 0.1 mol/l to an. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Two most important solutions used in iodometric titrations are solution. this titration process will use sodium thiosulfate (na2s2o3). This sodium thiosulfate is also known as a reducing agent to. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. solutions used in iodometric titrations. this titration process will use sodium thiosulfate (na2s2o3). 1.5 g of ki are. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From dxojsbiep.blob.core.windows.net
Iodometric Titration Of Iodine And Sodium Thiosulphate at Stacie King blog Why Is Sodium Thiosulfate Used In Iodometric Titration This sodium thiosulfate is also known as a reducing agent to titrate the iodine. 1.5 g of ki are added. this titration process will use sodium thiosulfate (na2s2o3). Then it is titrated with sodium thiosulfate 0.1 mol/l to an. solutions used in iodometric titrations. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From dxovbottd.blob.core.windows.net
Titration Experiment Notes at Victor Shoemaker blog Why Is Sodium Thiosulfate Used In Iodometric Titration Then it is titrated with sodium thiosulfate 0.1 mol/l to an. this titration process will use sodium thiosulfate (na2s2o3). solutions used in iodometric titrations. 1.5 g of ki are added. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From studylib.net
Salt Iodine Titration Method Why Is Sodium Thiosulfate Used In Iodometric Titration a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Two most important solutions used in iodometric titrations are solution. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. this titration process will use sodium thiosulfate (na2s2o3). solutions used in iodometric. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From mavink.com
Sodium Thiosulfate Titration Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). Two most important solutions used in iodometric titrations are solution. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. iodine. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From cezisuit.blob.core.windows.net
Redox Titration Using Sodium Thiosulphate Lab Report Discussion at Lee Palumbo blog Why Is Sodium Thiosulfate Used In Iodometric Titration iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. this titration process will use sodium thiosulfate (na2s2o3). arsenic oxide is dissolved in sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From mavink.com
Sodium Thiosulfate Titration Why Is Sodium Thiosulfate Used In Iodometric Titration This sodium thiosulfate is also known as a reducing agent to titrate the iodine. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From hyprowira.com
Iodometric Titration Functions and How It Works Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). Two most important solutions used in iodometric titrations are solution. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. solutions used in iodometric titrations. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. This sodium thiosulfate is also known as. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. Two most important solutions used in iodometric titrations are solution. solutions used in iodometric titrations. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. a precise and stable reducing agent, sodium thiosulfate. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From mavink.com
Sodium Thiosulfate Titration Why Is Sodium Thiosulfate Used In Iodometric Titration a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. 1.5 g of ki are added. this titration process will use sodium thiosulfate (na2s2o3). Then it is. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From syndel.com
Sodium thiosulfate Syndel Why Is Sodium Thiosulfate Used In Iodometric Titration iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. 1.5 g of ki are added. Two most important solutions used in iodometric titrations are solution. this titration process will use sodium thiosulfate (na2s2o3). . Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.youtube.com
N/10 Sodium Thiosulfate Solution Preparation and Standardization with K2Cr2O7 Iodometric Why Is Sodium Thiosulfate Used In Iodometric Titration iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Two most important solutions used in iodometric titrations are solution. 1.5 g of ki are added. this titration process will use sodium thiosulfate (na2s2o3). solutions used in iodometric titrations. Then it is titrated with sodium thiosulfate 0.1 mol/l. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.numerade.com
SOLVED Questions What is the purpose of each of the following reagents in this experiment? a Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with. Why Is Sodium Thiosulfate Used In Iodometric Titration.