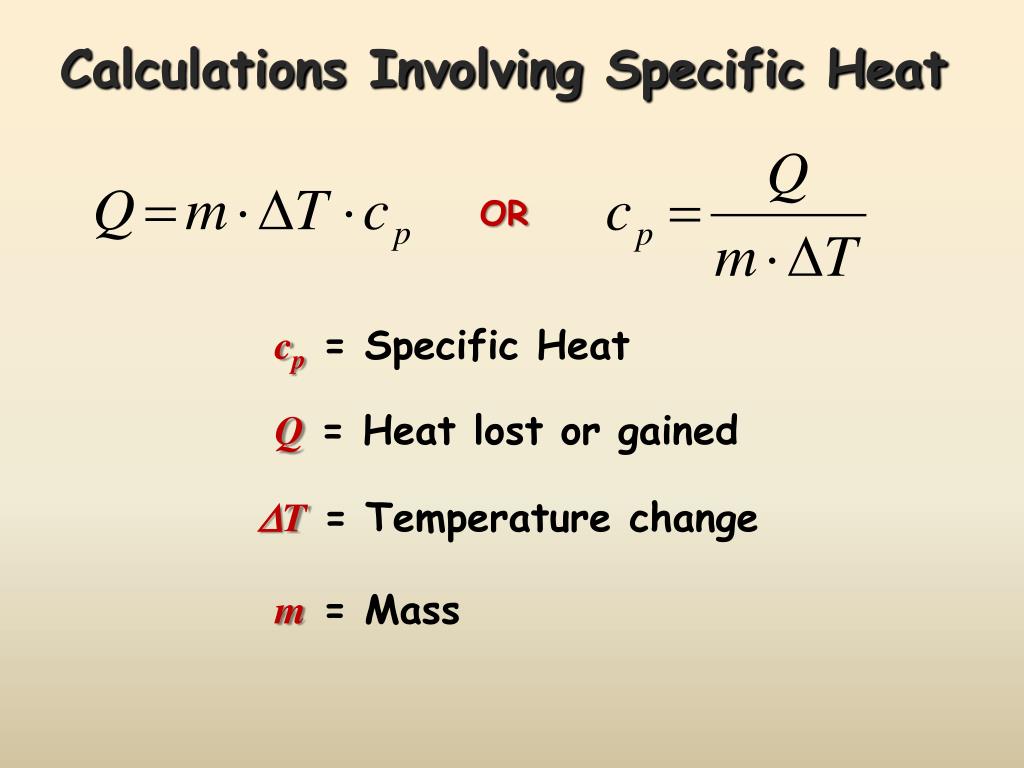
Calculating Heat Equation . use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. heat formula using heat capacity. To use calorimetric data to calculate enthalpy. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. calculate and interpret heat and related properties using typical calorimetry data.
from www.slideserve.com
One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. calculate and interpret heat and related properties using typical calorimetry data. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. heat formula using heat capacity. To use calorimetric data to calculate enthalpy. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is.
PPT Thermochemical Calculations PowerPoint Presentation, free
Calculating Heat Equation the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. calculate and interpret heat and related properties using typical calorimetry data. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. To use calorimetric data to calculate enthalpy. heat formula using heat capacity.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. the specific heat. Calculating Heat Equation.
From www.tessshebaylo.com
Use The Heat Equation To Calculate Energy In Joules And Calories For Calculating Heat Equation calculate and interpret heat and related properties using typical calorimetry data. heat formula using heat capacity. To use calorimetric data to calculate enthalpy. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost. Calculating Heat Equation.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation. Calculating Heat Equation.
From studylib.net
Heat Equation Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan,. Calculating Heat Equation.
From quizzlibhofmann.z19.web.core.windows.net
Equation For Specific Heat Calculating Heat Equation To use calorimetric data to calculate enthalpy. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in. Calculating Heat Equation.
From worksheetfullfunniest.z21.web.core.windows.net
Equation For Calculating Heat Calculating Heat Equation To use calorimetric data to calculate enthalpy. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. heat. Calculating Heat Equation.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for heat transfer \(q =. Calculating Heat Equation.
From www.grc.nasa.gov
Heat Transfer Calculating Heat Equation To use calorimetric data to calculate enthalpy. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. the. Calculating Heat Equation.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calculating Heat Equation the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation. Calculating Heat Equation.
From www.slideserve.com
PPT Thermochemical Calculations PowerPoint Presentation, free Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. calculate and interpret heat and related properties using typical calorimetry data. heat formula using heat capacity. One method of calculating the amount of heat transfer to. Calculating Heat Equation.
From www.tessshebaylo.com
Heat Loss Equation Chemistry Tessshebaylo Calculating Heat Equation To use calorimetric data to calculate enthalpy. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo. Calculating Heat Equation.
From quizzlistreplevies.z13.web.core.windows.net
Heat Required For Phase Change Formula Calculating Heat Equation One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation for heat transfer \(q =. Calculating Heat Equation.
From www.adicotengineering.com
Reheat/Heating Sizing Calculator Adicot, Inc. Calculating Heat Equation the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. To use calorimetric data. Calculating Heat Equation.
From worksheetlibrarysteins.z19.web.core.windows.net
Formula To Calculate Specific Heat Calculating Heat Equation One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. calculate and interpret heat and related properties using typical calorimetry data. To use calorimetric data to calculate enthalpy. heat formula using heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost. Calculating Heat Equation.
From www.slideserve.com
PPT ENERGY PowerPoint Presentation, free download ID3232966 Calculating Heat Equation the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. calculate and interpret heat and related properties using typical calorimetry data. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is.. Calculating Heat Equation.
From www.slideserve.com
PPT Change of State PowerPoint Presentation, free download ID1562618 Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan,. Calculating Heat Equation.
From www.chegg.com
Solved In this assignment, you will be calculating the heat Calculating Heat Equation calculate and interpret heat and related properties using typical calorimetry data. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. One method of calculating the amount of heat transfer to or from a substance is using. Calculating Heat Equation.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calculating Heat Equation One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. the specific heat of a substance can be used to calculate the temperature change that a. Calculating Heat Equation.
From www.youtube.com
Heat Transfer L12 p1 Finite Difference Heat Equation YouTube Calculating Heat Equation the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. heat formula using. Calculating Heat Equation.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. the specific heat. Calculating Heat Equation.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Calculating Heat Equation To use calorimetric data to calculate enthalpy. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo. Calculating Heat Equation.
From www.tessshebaylo.com
Equation For Energy Transferred Specific Heat Capacity Tessshebaylo Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. To use calorimetric data to calculate enthalpy. heat formula using heat capacity. calculate and interpret heat and related properties using typical calorimetry data. the specific. Calculating Heat Equation.
From studylib.net
Calculating Heat ANSWER KEY Calculating Heat Equation the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat. Calculating Heat Equation.
From www.tessshebaylo.com
Water Heating Equation Tessshebaylo Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for heat transfer \(q =. Calculating Heat Equation.
From www.grc.nasa.gov
Specific Heats Calorically Imperfect Gas Calculating Heat Equation the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by. Calculating Heat Equation.
From www.youtube.com
Calculating the Heat of the Solution YouTube Calculating Heat Equation One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. calculate and interpret heat and related properties using. Calculating Heat Equation.
From www.tessshebaylo.com
Water Heating Equation Tessshebaylo Calculating Heat Equation heat formula using heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. the specific. Calculating Heat Equation.
From studylib.net
Problem Set 1 Calculating Heat Calculating Heat Equation calculate and interpret heat and related properties using typical calorimetry data. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for. Calculating Heat Equation.
From learningmediajacob.z5.web.core.windows.net
Formula To Explain Specific Heat Calculating Heat Equation To use calorimetric data to calculate enthalpy. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. calculate and interpret heat and related properties using typical calorimetry data. the specific heat of a substance can be. Calculating Heat Equation.
From www.youtube.com
Solved example Calculating power & heat dissipated YouTube Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. calculate and interpret heat and related properties using typical calorimetry data. To use calorimetric data to calculate enthalpy. heat formula using heat capacity. use the. Calculating Heat Equation.
From www.youtube.com
GCSE Physics All Exam Boards Thermal Physics Specific Heat Calculating Heat Equation calculate and interpret heat and related properties using typical calorimetry data. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is. heat formula using heat capacity. To use calorimetric data to calculate enthalpy. the specific heat of a substance can be used to. Calculating Heat Equation.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Calculating Heat Equation heat formula using heat capacity. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific. Calculating Heat Equation.
From www.chegg.com
The timedependent heat equation in 1D space is a PDE Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. To use calorimetric data to calculate enthalpy. One method of calculating the amount of heat transfer to or from a substance is using its heat capacity. the. Calculating Heat Equation.
From www.youtube.com
Calculating Rate of Heat Transfer Between Two Working Fluids of a Heat Calculating Heat Equation heat formula using heat capacity. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when. Calculating Heat Equation.
From www.slideserve.com
PPT Heat, Temperature, and Internal Energy PowerPoint Presentation Calculating Heat Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan,. Calculating Heat Equation.