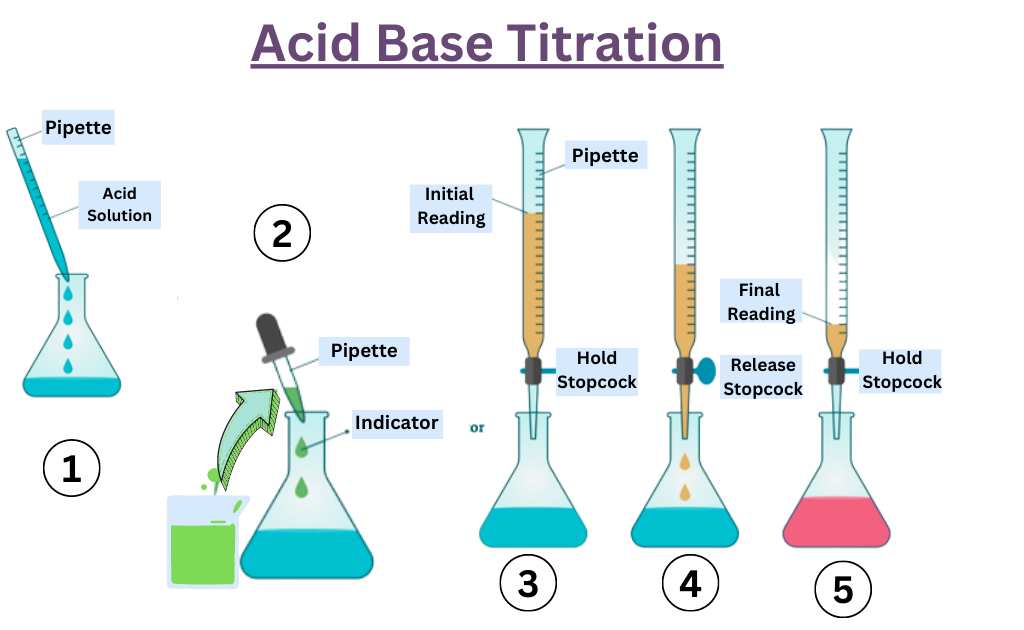
Titration Experiment Conclusion . Initially the ph is due to pure acetic acid. write your conclusion. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Includes kit list and safety. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the.
from hxeedchjz.blob.core.windows.net
write your conclusion. Initially the ph is due to pure acetic acid. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. Includes kit list and safety. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard.
Discussion Of Titration at Effie Hope blog
Titration Experiment Conclusion titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. Includes kit list and safety. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the ph is due to pure acetic acid. write your conclusion. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.
From exoxjybnj.blob.core.windows.net
Acid Base Titration Lab Introduction at Nichole Brumback blog Titration Experiment Conclusion write your conclusion. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is a process of measuring the concentration of an unknown solution by adding. Titration Experiment Conclusion.
From www.olsh.vic.edu.au
Newsletter Vol. 52 No. 9 14 September 2023 OLSH College Bentleigh Titration Experiment Conclusion Initially the ph is due to pure acetic acid. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. write your conclusion. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure. Titration Experiment Conclusion.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Experiment Conclusion Initially the ph is due to pure acetic acid. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. titration is a process of measuring the concentration of an unknown solution by adding a known. Titration Experiment Conclusion.
From www.slideshare.net
Titration Lab Report Titration Experiment Conclusion titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. a titration is an experiment where a volume of a solution of. Titration Experiment Conclusion.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Titration Experiment Conclusion In a titration, the conclusion is often a simple statement of the experimentally determined parameter. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is an analytical procedure used to determine the accurate concentration of. Titration Experiment Conclusion.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download Titration Experiment Conclusion write your conclusion. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the ph is due to pure acetic acid. In a titration, the conclusion is often a simple. Titration Experiment Conclusion.
From dxoaafwnm.blob.core.windows.net
Redox Titration Experiment Procedure at Margaret Campos blog Titration Experiment Conclusion write your conclusion. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. use this class practical to explore titration,. Titration Experiment Conclusion.
From hxeedchjz.blob.core.windows.net
Discussion Of Titration at Effie Hope blog Titration Experiment Conclusion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. a titration is an analytical procedure used to determine the accurate concentration of a. Titration Experiment Conclusion.
From acidsandbasesandmore.weebly.com
Titrations CHEMISTRY 101 Titration Experiment Conclusion Initially the ph is due to pure acetic acid. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. write your conclusion.. Titration Experiment Conclusion.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Experiment Conclusion In a titration, the conclusion is often a simple statement of the experimentally determined parameter. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. Includes kit list and safety. Initially the ph is due to pure acetic acid. use this class practical. Titration Experiment Conclusion.
From vasastephaniemackay.blogspot.com
Acid Base Titration Lab Report Answers Stephanie Mackay Titration Experiment Conclusion In a titration, the conclusion is often a simple statement of the experimentally determined parameter. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. write your conclusion. Initially the ph is due to pure acetic acid. titration of acetic acid with. Titration Experiment Conclusion.
From www.studocu.com
Experiment 7 Titrations Experiment 7 Titrations Required reading Titration Experiment Conclusion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Initially the ph is due to pure acetic acid. a titration is an analytical procedure used to determine the accurate concentration of a sample by. Titration Experiment Conclusion.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment Conclusion Initially the ph is due to pure acetic acid. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. write your. Titration Experiment Conclusion.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Titration Experiment Conclusion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another. Titration Experiment Conclusion.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Titration Experiment Conclusion Includes kit list and safety. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. write your conclusion. use this class. Titration Experiment Conclusion.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Titration Experiment Conclusion a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Includes kit list and safety. titration is a process of measuring the concentration of an unknown solution by. Titration Experiment Conclusion.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Titration Experiment Conclusion Includes kit list and safety. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. The purpose of the titration is to find the concentration of the hydrochloric acid. Titration Experiment Conclusion.
From exogoqrkx.blob.core.windows.net
Titration Class 12Th Practical at Dolores Parker blog Titration Experiment Conclusion Initially the ph is due to pure acetic acid. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting. Titration Experiment Conclusion.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Experiment Conclusion titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Includes kit list and safety. titration. Titration Experiment Conclusion.
From dxovbottd.blob.core.windows.net
Titration Experiment Notes at Victor Shoemaker blog Titration Experiment Conclusion titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the ph is due to pure acetic acid. The purpose of the titration is to find the concentration of the hydrochloric. Titration Experiment Conclusion.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Titration Experiment Conclusion write your conclusion. Includes kit list and safety. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. The purpose of the titration is to find the concentration. Titration Experiment Conclusion.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Titration Experiment Conclusion titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. Initially the ph is due to pure acetic acid. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. In. Titration Experiment Conclusion.
From www.slideshare.net
Titration experiment report Titration Experiment Conclusion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. write your conclusion. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Includes kit list and safety. titration is a process of measuring the concentration of. Titration Experiment Conclusion.
From dxopjnnhs.blob.core.windows.net
A Level Chemistry Core Practical 11 Redox Titration at Abby Moseley blog Titration Experiment Conclusion a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Initially the ph is due to pure acetic acid. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. titration of. Titration Experiment Conclusion.
From www.slideshare.net
Titration report Titration Experiment Conclusion Includes kit list and safety. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. write your conclusion. Initially the ph. Titration Experiment Conclusion.
From studylib.net
Experiment 1 Titration of Amino Acids Titration Experiment Conclusion titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. write your conclusion. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is. Titration Experiment Conclusion.
From mungfali.com
Acid Base Titration Experiment Titration Experiment Conclusion a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. Initially. Titration Experiment Conclusion.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co Titration Experiment Conclusion Includes kit list and safety. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard.. Titration Experiment Conclusion.
From www.slideshare.net
Acid base titration Titration Experiment Conclusion In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Includes kit list and safety. write your conclusion. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The purpose of the titration is to find the concentration of the hydrochloric acid. Titration Experiment Conclusion.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Experiment Conclusion The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. use this class practical to explore titration, producing the salt sodium chloride. Titration Experiment Conclusion.
From de.vecteezy.com
Acid Base Titration Experiment und Phasen von Farbe Veränderung während Titration Experiment Conclusion Includes kit list and safety. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Initially the ph is due to pure acetic acid. titration is a process of measuring the. Titration Experiment Conclusion.
From www.studocu.com
Experiment 20G primary standards and Titrations Conclusion The Titration Experiment Conclusion titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Includes kit list and safety. Initially the ph. Titration Experiment Conclusion.
From www.chegg.com
Solved Experiment 17B AcidBase Titration OBJECTIVES In Titration Experiment Conclusion Initially the ph is due to pure acetic acid. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. titration is a process of measuring the concentration of. Titration Experiment Conclusion.
From dxoudnvol.blob.core.windows.net
What Is The Purpose Of Titration Experiment at Andrew Peterson blog Titration Experiment Conclusion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. titration of acetic acid. Titration Experiment Conclusion.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Experiment Conclusion use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the. a titration is an experiment where a volume of a solution of known concentration. Titration Experiment Conclusion.