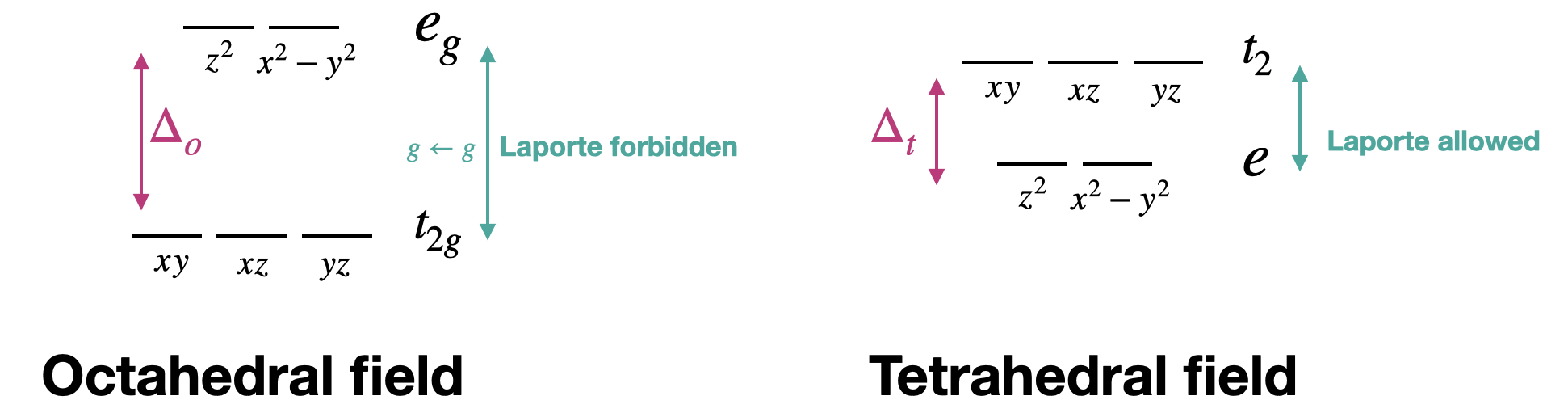
D-D Transition Selection Rules . So finally, for hydrogen, we have the following selection rules: The spin selection rule, δs = 0. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. I.e., an octahedral “ligand field”. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The integrated intensity or oscillator. The 6 ligands are put on the x, y, z axes (black dots. Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. The selection rules governing transitions between electronic energy levels of transition metal complexes are: Δs = 0 the spin rule.
from chem.libretexts.org
The 6 ligands are put on the x, y, z axes (black dots. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. So finally, for hydrogen, we have the following selection rules: In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: I.e., an octahedral “ligand field”. Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. The selection rules governing transitions between electronic energy levels of transition metal complexes are: The spin selection rule, δs = 0.
11.3.6 Tetrahedral Complexes Chemistry LibreTexts
D-D Transition Selection Rules Selection rules for electronic transitions determine whether a transition is allowed or forbidden. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. Δs = 0 the spin rule. The selection rules governing transitions between electronic energy levels of transition metal complexes are: The spin selection rule, δs = 0. The 6 ligands are put on the x, y, z axes (black dots. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: So finally, for hydrogen, we have the following selection rules: I.e., an octahedral “ligand field”. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The integrated intensity or oscillator.
From scoop.eduncle.com
Which rule is dominant.,spin selection rule or laporte? D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels (including microstates and terms) are: The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels of transition metal complexes are: Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example. D-D Transition Selection Rules.
From www.slideserve.com
PPT Electronic Spectra of Coordination Compounds PowerPoint D-D Transition Selection Rules Selection rules for electronic transitions determine whether a transition is allowed or forbidden. Δs = 0 the spin rule. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: I.e., an octahedral “ligand field”. The 6 ligands are put on the x, y, z axes (black dots. So finally, for hydrogen, we have the following selection. D-D Transition Selection Rules.
From www.slideserve.com
PPT Colour and electronic spectra PowerPoint Presentation ID2019794 D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Δs = 0 the spin rule. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. So finally, for hydrogen, we have the following selection rules: The selection rules governing transitions between electronic energy levels of transition. D-D Transition Selection Rules.
From www.youtube.com
Spin & Laporte selection rules for dd transition YouTube D-D Transition Selection Rules Δs = 0 the spin rule. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The 6 ligands are put on the x, y, z axes (black dots. The selection rules governing transitions between electronic energy levels of transition metal complexes are: The spin selection rule, δs = 0. So. D-D Transition Selection Rules.
From chem.libretexts.org
11.3.6 Tetrahedral Complexes Chemistry LibreTexts D-D Transition Selection Rules So finally, for hydrogen, we have the following selection rules: Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The integrated intensity or oscillator. The 6 ligands are put on the x, y, z axes (black dots. The selection rules governing transitions between electronic energy levels of transition metal complexes are: I.e., an octahedral “ligand field”.. D-D Transition Selection Rules.
From www.researchgate.net
(a) Transition selection rules dictated by the threefold inplane D-D Transition Selection Rules Selection rules for electronic transitions determine whether a transition is allowed or forbidden. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. So finally, for hydrogen, we have the following selection rules: The 6 ligands are put on the x, y, z axes (black dots. The spin selection rule, δs. D-D Transition Selection Rules.
From www.numerade.com
SOLVED The electronic configuration and geometry of a complex D-D Transition Selection Rules Δs = 0 the spin rule. Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: The selection rules governing transitions between electronic energy levels. D-D Transition Selection Rules.
From www.youtube.com
Selection Rules for Electron Transitions YouTube D-D Transition Selection Rules I.e., an octahedral “ligand field”. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Δs = 0 the spin rule. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. Δ j = 0 , ± 1 , δ m j = 0 , ± 1. D-D Transition Selection Rules.
From www.youtube.com
20 6 Selection Rules Why dd Transitions Are Observed YouTube D-D Transition Selection Rules The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels of transition metal complexes are: The spin selection rule, δs = 0. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The 6. D-D Transition Selection Rules.
From www.youtube.com
Electron Spectra of Transition Metal Complexes Selection Rules dd D-D Transition Selection Rules The spin selection rule, δs = 0. The selection rules governing transitions between electronic energy levels of transition metal complexes are: Δs = 0 the spin rule. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l =. D-D Transition Selection Rules.
From www.youtube.com
Transition ElementsColour (dd Transition)chemistry science pmc D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Δs = 0 the spin rule. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The selection rules governing transitions between electronic energy. D-D Transition Selection Rules.
From www.slideserve.com
PPT The Electronic Spectra of Coordination Compounds PowerPoint D-D Transition Selection Rules The 6 ligands are put on the x, y, z axes (black dots. The spin selection rule, δs = 0. The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Δs = 0 the spin rule. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. I.e., an. D-D Transition Selection Rules.
From www.youtube.com
dd Transition Part 34 Unit 9 CBSE grade 12 chemistry coordination D-D Transition Selection Rules The 6 ligands are put on the x, y, z axes (black dots. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The spin selection rule, δs = 0. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The integrated intensity or oscillator. Δ j =. D-D Transition Selection Rules.
From www.slideserve.com
PPT Colour and electronic spectra PowerPoint Presentation, free D-D Transition Selection Rules In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. So finally, for hydrogen, we have the following selection rules:. D-D Transition Selection Rules.
From www.doubtnut.com
Which of the following ions exhibits dd transitions and D-D Transition Selection Rules Δs = 0 the spin rule. The spin selection rule, δs = 0. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The 6 ligands are put on the x, y, z axes (black dots. So finally, for hydrogen, we have the following selection rules: The integrated intensity or oscillator.. D-D Transition Selection Rules.
From scoop.eduncle.com
The number of dd transition(s) expected for the complex [cu(nh3)2(h;0 D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels (including microstates and terms) are: In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. So finally, for hydrogen, we have the following selection rules: I.e., an octahedral “ligand field”. Selection rules for electronic transitions determine whether a transition is allowed or. D-D Transition Selection Rules.
From www.youtube.com
🔥 Selection Rule For dd transition 💡 dd transition 🔥 d d transition 🔥 D-D Transition Selection Rules In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. I.e., an octahedral “ligand field”. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Δ j = 0 , ± 1 , δ. D-D Transition Selection Rules.
From www.slideserve.com
PPT Lecture 36 Electronic spectroscopy PowerPoint Presentation ID D-D Transition Selection Rules In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The integrated intensity or oscillator. I.e., an octahedral “ligand field”. So finally, for hydrogen, we have the following selection rules: Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ±. D-D Transition Selection Rules.
From www.slideserve.com
PPT Motivation PowerPoint Presentation, free download ID4418328 D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The spin selection rule, δs = 0. I.e., an octahedral “ligand field”. The selection rules governing transitions between electronic energy levels of transition metal complexes are: The integrated intensity or oscillator. In order. D-D Transition Selection Rules.
From www.slideserve.com
PPT Electronic Spectroscopy PowerPoint Presentation, free download D-D Transition Selection Rules I.e., an octahedral “ligand field”. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. So finally, for hydrogen, we have the following selection rules: Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the. D-D Transition Selection Rules.
From www.youtube.com
dd संक्रमण,dd Transition,चक्रण चयन नियम,Spin selection Rule, unit8 D-D Transition Selection Rules The integrated intensity or oscillator. So finally, for hydrogen, we have the following selection rules: I.e., an octahedral “ligand field”. Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. The spin selection rule, δs = 0. Δs =. D-D Transition Selection Rules.
From www.youtube.com
Colour due to dd Transition Complementary colour scheme Trick D-D Transition Selection Rules The 6 ligands are put on the x, y, z axes (black dots. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: So finally, for hydrogen, we have the following selection rules: I.e., an octahedral “ligand field”. The selection rules governing transitions between electronic energy levels of transition metal complexes are: Selection rules for electronic. D-D Transition Selection Rules.
From www.slideserve.com
PPT Electronic Spectra of Coordination Compounds PowerPoint D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels of transition metal complexes are: Δs = 0 the spin rule. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The spin selection rule, δs = 0. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. The. D-D Transition Selection Rules.
From slidetodoc.com
The roles of orbital in the optical and D-D Transition Selection Rules So finally, for hydrogen, we have the following selection rules: I.e., an octahedral “ligand field”. The selection rules governing transitions between electronic energy levels of transition metal complexes are: The spin selection rule, δs = 0. Δs = 0 the spin rule. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be. D-D Transition Selection Rules.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy D-D Transition Selection Rules The integrated intensity or oscillator. So finally, for hydrogen, we have the following selection rules: The spin selection rule, δs = 0. The 6 ligands are put on the x, y, z axes (black dots. Δs = 0 the spin rule. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. I.e., an octahedral “ligand field”. In. D-D Transition Selection Rules.
From www.youtube.com
Selection rules and d to d transitions YouTube D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels (including microstates and terms) are: Δs = 0 the spin rule. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. So finally, for hydrogen, we have the following selection rules: I.e., an octahedral “ligand field”. The spin selection rule, δs = 0. The selection rules governing transitions. D-D Transition Selection Rules.
From www.slideserve.com
PPT Periodicity (AHL) PowerPoint Presentation ID2249355 D-D Transition Selection Rules Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels of transition metal complexes are: Selection rules for electronic transitions determine whether a transition is. D-D Transition Selection Rules.
From www.youtube.com
Bands of dd transition Weaker, Broader YouTube D-D Transition Selection Rules Δs = 0 the spin rule. So finally, for hydrogen, we have the following selection rules: The selection rules governing transitions between electronic energy levels of transition metal complexes are: Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay. D-D Transition Selection Rules.
From slideplayer.com
Light (EM Radiation) Characteristics ppt download D-D Transition Selection Rules Δs = 0 the spin rule. So finally, for hydrogen, we have the following selection rules: The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels (including microstates and terms) are: I.e., an octahedral “ligand field”. The 6 ligands are put on the x, y, z axes (black dots. The selection rules governing transitions between electronic. D-D Transition Selection Rules.
From chem.libretexts.org
Selection rules and transition moment integral Chemistry LibreTexts D-D Transition Selection Rules The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels of transition metal complexes are: Δs = 0 the spin rule. The spin selection rule, δs = 0. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. I.e., an octahedral “ligand field”. So finally, for hydrogen,. D-D Transition Selection Rules.
From studylib.net
Selection Rules for electronic transitions D-D Transition Selection Rules The spin selection rule, δs = 0. So finally, for hydrogen, we have the following selection rules: Δ j = 0 , ± 1 , δ m j = 0 , ± 1 and δ l = ± 1 we saw from the example of the decay of. I.e., an octahedral “ligand field”. Selection rules for electronic transitions determine whether. D-D Transition Selection Rules.
From www.slideserve.com
PPT Coordination Chemistry III Electronic Spectra PowerPoint D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels of transition metal complexes are: The 6 ligands are put on the x, y, z axes (black dots. So finally, for hydrogen, we have the following selection rules: Δs = 0 the spin rule. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must. D-D Transition Selection Rules.
From www.slideserve.com
PPT Nuclear deexcitation PowerPoint Presentation, free download ID D-D Transition Selection Rules The selection rules governing transitions between electronic energy levels (including microstates and terms) are: The 6 ligands are put on the x, y, z axes (black dots. Selection rules for electronic transitions determine whether a transition is allowed or forbidden. The selection rules governing transitions between electronic energy levels of transition metal complexes are: In order for an electronic transition. D-D Transition Selection Rules.
From www.youtube.com
B.Sc. III Year Selection rules for dd transitions dd संक्रमण के D-D Transition Selection Rules I.e., an octahedral “ligand field”. The selection rules governing transitions between electronic energy levels of transition metal complexes are: Δs = 0 the spin rule. The 6 ligands are put on the x, y, z axes (black dots. So finally, for hydrogen, we have the following selection rules: The selection rules governing transitions between electronic energy levels (including microstates and. D-D Transition Selection Rules.
From chem.libretexts.org
Selection rules and transition moment integral Chemistry LibreTexts D-D Transition Selection Rules I.e., an octahedral “ligand field”. In order for an electronic transition to be allowed (occur with strong intensity), certain selection rules must be obeyed. So finally, for hydrogen, we have the following selection rules: The spin selection rule, δs = 0. The integrated intensity or oscillator. The selection rules governing transitions between electronic energy levels of transition metal complexes are:. D-D Transition Selection Rules.