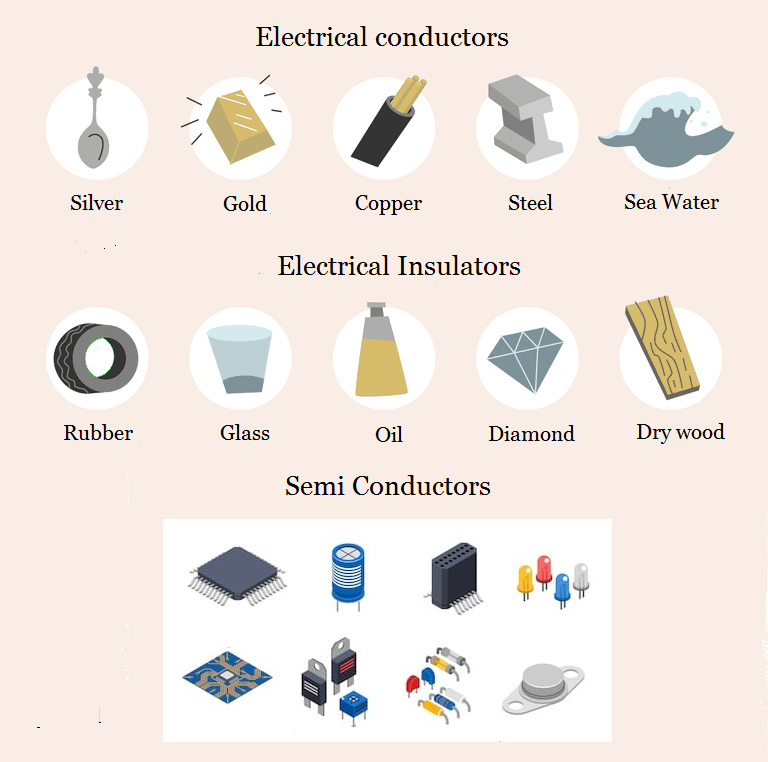
Electrical Conductor Non Metals Examples . Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. Only metals are electrical conductors. Water as it can contain dissolved minerals. Only metals are electrical conductors. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Examples of electrical insulators are plastic, rubber, wood, glass and air. They tend not to be malleable or ductile, so they form brittle solids. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. For example, carbon in the form of graphite is an excellent conductor of electricity. The centre of most pencils contains graphite. This leaves one electron free for bonding.
from mavink.com
Only metals are electrical conductors. Water as it can contain dissolved minerals. This leaves one electron free for bonding. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; For example, carbon in the form of graphite is an excellent conductor of electricity. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. The centre of most pencils contains graphite. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. They tend not to be malleable or ductile, so they form brittle solids. Examples of electrical insulators are plastic, rubber, wood, glass and air.
Examples Of Conductors And Insulators At Home
Electrical Conductor Non Metals Examples Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. Only metals are electrical conductors. The centre of most pencils contains graphite. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. For example, carbon in the form of graphite is an excellent conductor of electricity. Examples of electrical insulators are plastic, rubber, wood, glass and air. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Only metals are electrical conductors. Water as it can contain dissolved minerals. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; They tend not to be malleable or ductile, so they form brittle solids. This leaves one electron free for bonding.
From sciencenotes.org
What Is the Most Conductive Element? Electrical Conductor Non Metals Examples If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Water as it can contain dissolved minerals. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; For example, carbon in the form. Electrical Conductor Non Metals Examples.
From www.youtube.com
ELECTRICAL CONDUCTIVITY OF METALS AND NON METALS SCIENCE EXPERIMENT Electrical Conductor Non Metals Examples Only metals are electrical conductors. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Examples of electrical insulators are plastic, rubber, wood,. Electrical Conductor Non Metals Examples.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Field PowerPoint Electrical Conductor Non Metals Examples In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; The centre of most pencils contains graphite. Examples of electrical insulators are plastic, rubber, wood, glass and air. Only metals are electrical conductors. This leaves one electron free for bonding. If. Electrical Conductor Non Metals Examples.
From nittygrittyscience.com
Section 1 Electricity Nitty Gritty Science Electrical Conductor Non Metals Examples In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. Examples of electrical insulators are plastic, rubber, wood, glass and air. Water as it can contain. Electrical Conductor Non Metals Examples.
From scienceislife4sgss.weebly.com
Electricity and Lighting SCIENCE IS LIFE Electrical Conductor Non Metals Examples They tend not to be malleable or ductile, so they form brittle solids. Examples of electrical insulators are plastic, rubber, wood, glass and air. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Water as it. Electrical Conductor Non Metals Examples.
From www.electricaltechnology.org
Types of Electrical Wires and Cables Electrical Technology Electrical Conductor Non Metals Examples Only metals are electrical conductors. Water as it can contain dissolved minerals. The centre of most pencils contains graphite. They tend not to be malleable or ductile, so they form brittle solids. For example, carbon in the form of graphite is an excellent conductor of electricity. If you see the structure of graphite, only three of the four carbon atoms. Electrical Conductor Non Metals Examples.
From in.pinterest.com
Conductors and insulators Insulation, Electricity, Conductors Electrical Conductor Non Metals Examples Water as it can contain dissolved minerals. They tend not to be malleable or ductile, so they form brittle solids. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Nonmetals are typically poor conductors of heat and electricity, with low. Electrical Conductor Non Metals Examples.
From www.flexiprep.com
NCERT Class VIII Science Solutions Chapter 4 Materials Metals and Non Electrical Conductor Non Metals Examples Only metals are electrical conductors. Only metals are electrical conductors. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. The centre of most pencils contains graphite. They tend not to be malleable or ductile, so they form brittle solids. This leaves one electron free for bonding. If you see the structure of graphite, only. Electrical Conductor Non Metals Examples.
From www.reagent.co.uk
What Is Conductivity In Chemistry? The Science Blog Electrical Conductor Non Metals Examples For example, carbon in the form of graphite is an excellent conductor of electricity. Examples of electrical insulators are plastic, rubber, wood, glass and air. They tend not to be malleable or ductile, so they form brittle solids. Water as it can contain dissolved minerals. In a conductor, the valence band is partially filled, and since there are numerous empty. Electrical Conductor Non Metals Examples.
From www.slideserve.com
PPT L 23 Electricity & [1] PowerPoint Presentation ID6390016 Electrical Conductor Non Metals Examples Only metals are electrical conductors. The centre of most pencils contains graphite. Water as it can contain dissolved minerals. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Examples of electrical insulators are plastic, rubber, wood, glass and air. They tend not to be malleable or ductile, so they form brittle. Electrical Conductor Non Metals Examples.
From www.thoughtco.com
List of the Best Conductors of Electricity Electrical Conductor Non Metals Examples Only metals are electrical conductors. The centre of most pencils contains graphite. For example, carbon in the form of graphite is an excellent conductor of electricity. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Water as it can contain. Electrical Conductor Non Metals Examples.
From ksa.mytutorsource.com
Electrical Conductor, Electrical Insulator, And Thermal Conductor Electrical Conductor Non Metals Examples Only metals are electrical conductors. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Examples of electrical insulators are plastic, rubber, wood, glass and air. For example, carbon in the form of graphite is an excellent conductor of electricity. Only. Electrical Conductor Non Metals Examples.
From eepower.com
Understanding the Electrical Properties of Conductors, Insulators, and Electrical Conductor Non Metals Examples Only metals are electrical conductors. This leaves one electron free for bonding. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Examples of electrical insulators are plastic, rubber, wood, glass and air. Only metals are electrical. Electrical Conductor Non Metals Examples.
From www.ny-engineers.com
Selecting the Right Type of Electrical Raceway Nonmetallic Conduit Options Electrical Conductor Non Metals Examples In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Water as it can contain dissolved minerals. Only metals are electrical conductors. The centre of most pencils contains graphite. They tend not to be malleable or ductile, so they form brittle. Electrical Conductor Non Metals Examples.
From mavink.com
Examples Of Conductors And Insulators At Home Electrical Conductor Non Metals Examples This leaves one electron free for bonding. Water as it can contain dissolved minerals. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. Only metals are electrical conductors. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of. Electrical Conductor Non Metals Examples.
From sciencenotes.org
Metals vs Nonmetals Electrical Conductor Non Metals Examples Only metals are electrical conductors. Examples of electrical insulators are plastic, rubber, wood, glass and air. The centre of most pencils contains graphite. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Water as it can contain dissolved minerals. This. Electrical Conductor Non Metals Examples.
From exobmjpvb.blob.core.windows.net
Electrical Conductor Is The Best at Theresa Moore blog Electrical Conductor Non Metals Examples Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. The centre of most pencils contains graphite. This leaves one electron free for bonding. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Only metals. Electrical Conductor Non Metals Examples.
From sciencenotes.org
Examples of Conductors and Insulators Electrical Conductor Non Metals Examples Only metals are electrical conductors. For example, carbon in the form of graphite is an excellent conductor of electricity. They tend not to be malleable or ductile, so they form brittle solids. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Nonmetals are typically poor conductors of heat and electricity, with. Electrical Conductor Non Metals Examples.
From www.youtube.com
Why metals conduct electricity and non metals does not conduct Electrical Conductor Non Metals Examples They tend not to be malleable or ductile, so they form brittle solids. Only metals are electrical conductors. Only metals are electrical conductors. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move. Electrical Conductor Non Metals Examples.
From www.slideshare.net
Chapter 24 Conduction Electrical Conductor Non Metals Examples If you see the structure of graphite, only three of the four carbon atoms are used for bonding. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. This leaves one electron free for bonding. Examples of electrical insulators are plastic, rubber, wood, glass and air. The centre of most pencils contains graphite. In a. Electrical Conductor Non Metals Examples.
From punchlistzero.com
NonConductive Metals Examples and Uses Electrical Conductor Non Metals Examples This leaves one electron free for bonding. They tend not to be malleable or ductile, so they form brittle solids. Examples of electrical insulators are plastic, rubber, wood, glass and air. Only metals are electrical conductors. Water as it can contain dissolved minerals. The centre of most pencils contains graphite. For example, carbon in the form of graphite is an. Electrical Conductor Non Metals Examples.
From slideplayer.com
Properties of Material Grade 10 Physical Science Mrs K Faling ppt Electrical Conductor Non Metals Examples Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. Only metals are electrical conductors. Only metals are electrical conductors. Examples of electrical insulators are plastic, rubber, wood, glass and air. This leaves one electron free for bonding. They tend not to be malleable or ductile, so they form brittle solids. For example, carbon in. Electrical Conductor Non Metals Examples.
From materialmediasclates.z21.web.core.windows.net
Electrical Conductors And Insulators Worksheet Electrical Conductor Non Metals Examples The centre of most pencils contains graphite. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. This leaves one electron free for bonding. They tend not to be malleable or ductile, so they form brittle solids. For example, carbon in the form of graphite is an excellent conductor of electricity. In. Electrical Conductor Non Metals Examples.
From www.electricalonline4u.com
What are Conductors and Insulators? Guide Electrical Conductor Non Metals Examples Only metals are electrical conductors. For example, carbon in the form of graphite is an excellent conductor of electricity. Water as it can contain dissolved minerals. The centre of most pencils contains graphite. They tend not to be malleable or ductile, so they form brittle solids. If you see the structure of graphite, only three of the four carbon atoms. Electrical Conductor Non Metals Examples.
From www.slideserve.com
PPT L 24 Electricity & [1] PowerPoint Presentation ID2647184 Electrical Conductor Non Metals Examples In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; The centre of most pencils contains graphite. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. Only metals are electrical conductors. Only metals are electrical. Electrical Conductor Non Metals Examples.
From studyzonehahunsticking.z21.web.core.windows.net
Electrical Conductors And Insulators Grade 6 Electrical Conductor Non Metals Examples Water as it can contain dissolved minerals. This leaves one electron free for bonding. For example, carbon in the form of graphite is an excellent conductor of electricity. The centre of most pencils contains graphite. Only metals are electrical conductors. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free. Electrical Conductor Non Metals Examples.
From www.youtube.com
Metals and Nonmetals Conductors of heat and electricity Home Electrical Conductor Non Metals Examples For example, carbon in the form of graphite is an excellent conductor of electricity. Water as it can contain dissolved minerals. This leaves one electron free for bonding. Examples of electrical insulators are plastic, rubber, wood, glass and air. The centre of most pencils contains graphite. In a conductor, the valence band is partially filled, and since there are numerous. Electrical Conductor Non Metals Examples.
From www.thoughtco.com
10 Examples of Electrical Conductors and Insulators Electrical Conductor Non Metals Examples Only metals are electrical conductors. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. The centre of most pencils contains graphite. Water as it can contain dissolved minerals. Examples of electrical insulators are plastic, rubber, wood, glass and air. For example, carbon in the form of graphite is an excellent conductor of electricity. If. Electrical Conductor Non Metals Examples.
From studyzonehahunsticking.z21.web.core.windows.net
Electrical Conductors And Insulators Grade 6 Electrical Conductor Non Metals Examples Examples of electrical insulators are plastic, rubber, wood, glass and air. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Only metals are electrical conductors. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points.. Electrical Conductor Non Metals Examples.
From studylib.net
Conductors and Insulators rosedalegrade9electricity Electrical Conductor Non Metals Examples Examples of electrical insulators are plastic, rubber, wood, glass and air. Water as it can contain dissolved minerals. This leaves one electron free for bonding. Only metals are electrical conductors. Only metals are electrical conductors. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence. Electrical Conductor Non Metals Examples.
From www.geeksforgeeks.org
What is Conductor ? Electrical Conductor Non Metals Examples Water as it can contain dissolved minerals. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; If you see the structure of graphite, only three. Electrical Conductor Non Metals Examples.
From www.thoughtco.com
Electrical Conductivity of Metals Electrical Conductor Non Metals Examples For example, carbon in the form of graphite is an excellent conductor of electricity. Only metals are electrical conductors. This leaves one electron free for bonding. Water as it can contain dissolved minerals. Only metals are electrical conductors. Examples of electrical insulators are plastic, rubber, wood, glass and air. The centre of most pencils contains graphite. If you see the. Electrical Conductor Non Metals Examples.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID6856680 Electrical Conductor Non Metals Examples Water as it can contain dissolved minerals. For example, carbon in the form of graphite is an excellent conductor of electricity. Examples of electrical insulators are plastic, rubber, wood, glass and air. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. Only metals are electrical conductors. The centre of most pencils contains graphite. They. Electrical Conductor Non Metals Examples.
From www.physicsfox.org
What is Electricity? • Electricity • Physics Fox Electrical Conductor Non Metals Examples The centre of most pencils contains graphite. This leaves one electron free for bonding. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; Only metals are electrical conductors. Only metals are electrical conductors. Nonmetals are typically poor conductors of heat. Electrical Conductor Non Metals Examples.
From howtofunda.com
electricity working model science project on conductor or non conductor Electrical Conductor Non Metals Examples This leaves one electron free for bonding. Examples of electrical insulators are plastic, rubber, wood, glass and air. Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. If you see the structure of graphite, only three of the four carbon atoms are used for bonding. They tend not to be malleable or ductile, so. Electrical Conductor Non Metals Examples.