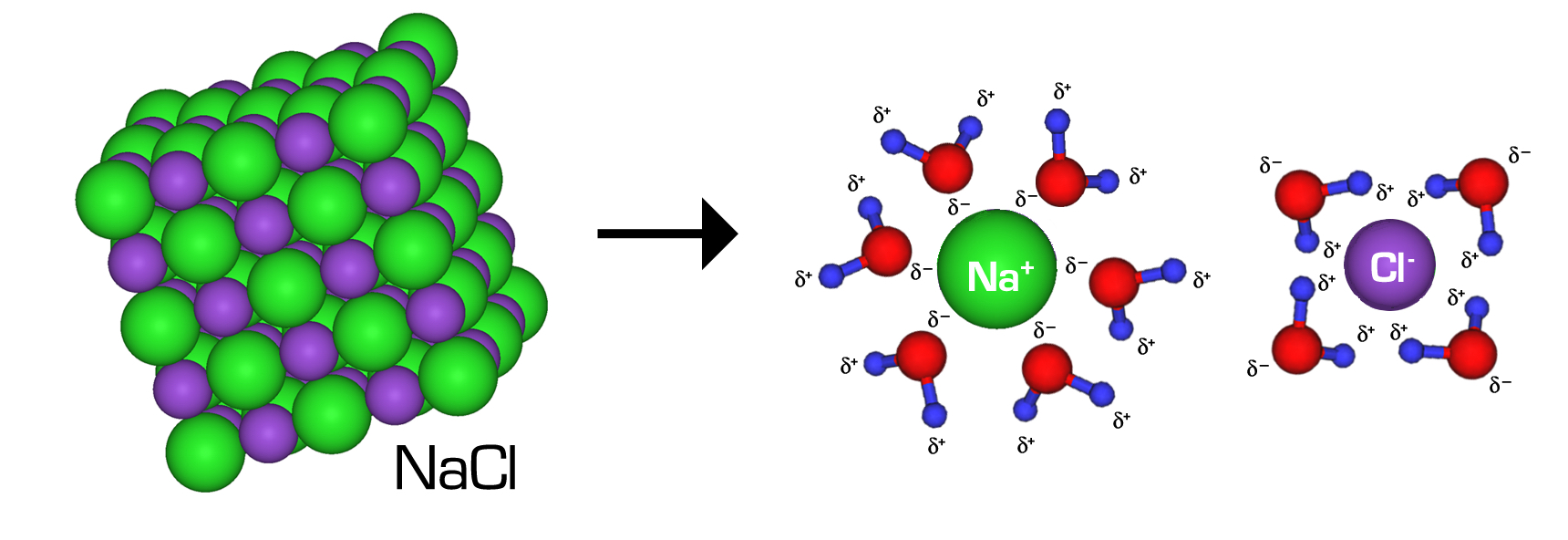
What Are Ionic Compounds Known As When Dissolved In Water . study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. For example, sodium chloride (nacl). study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. learn how ionic compounds dissociate and disperse in water to form electrolytes. Find out which compounds are.
from www.visionlearning.com
when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. For example, sodium chloride (nacl). when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. learn how ionic compounds dissociate and disperse in water to form electrolytes. Find out which compounds are. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal.
Solutions, Solubility, and Colligative Properties Chemistry
What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. For example, sodium chloride (nacl). study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. Find out which compounds are. learn how ionic compounds dissociate and disperse in water to form electrolytes. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2948816 What Are Ionic Compounds Known As When Dissolved In Water learn how ionic compounds dissociate and disperse in water to form electrolytes. For example, sodium chloride (nacl). when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. when ionic compounds dissolve in water, the. What Are Ionic Compounds Known As When Dissolved In Water.
From israelyouthsims.blogspot.com
Best Describes the Properties of Ionic Compounds What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. For example, sodium chloride (nacl). Find out which compounds are. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. when ionic compounds are dissolved into water, the polar water molecules. What Are Ionic Compounds Known As When Dissolved In Water.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic compounds dissolve in water, the ions. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download What Are Ionic Compounds Known As When Dissolved In Water learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. Find out which compounds are. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. study with. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Find out which compounds are. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. study with. What Are Ionic Compounds Known As When Dissolved In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Solubility Do Now p.4 PowerPoint Presentation, free download What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. when ionic compounds dissolve in water, the ions in the solid. What Are Ionic Compounds Known As When Dissolved In Water.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 What Are Ionic Compounds Known As When Dissolved In Water learn how ionic compounds dissociate and disperse in water to form electrolytes. Find out which compounds are. For example, sodium chloride (nacl). when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. . What Are Ionic Compounds Known As When Dissolved In Water.
From www.bharatagritech.com
Ions In Aqueous Solution Infographic Diagram Showing, 54 OFF What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. For example, sodium chloride (nacl). when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions.. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. study with quizlet and memorize flashcards containing terms. What Are Ionic Compounds Known As When Dissolved In Water.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts What Are Ionic Compounds Known As When Dissolved In Water Find out which compounds are. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. . What Are Ionic Compounds Known As When Dissolved In Water.
From circuitdbhomemade.z13.web.core.windows.net
Kcl Dissolved In Water Diagram What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. learn how ionic compounds dissociate and disperse in water to form electrolytes. study with quizlet and memorize flashcards containing terms like what determines whether. What Are Ionic Compounds Known As When Dissolved In Water.
From www.studocu.com
Solubility of ionic compounds H2SO4) total ionic equations are What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. study with quizlet and memorize flashcards. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID2732051 What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. For example, sodium chloride (nacl). study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. . What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 What Are Ionic Compounds Known As When Dissolved In Water learn how ionic compounds dissociate and disperse in water to form electrolytes. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. For example, sodium chloride (nacl). when ionic compounds. What Are Ionic Compounds Known As When Dissolved In Water.
From opentextbc.ca
Ionic Equations A Closer Look Introductory Chemistry, 1st Canadian What Are Ionic Compounds Known As When Dissolved In Water For example, sodium chloride (nacl). when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Find out which compounds are. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed. What Are Ionic Compounds Known As When Dissolved In Water.
From 2012books.lardbucket.org
The Dissolution Process What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Find out which compounds are. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. learn how. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation What Are Ionic Compounds Known As When Dissolved In Water Find out which compounds are. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds are dissolved into water, the polar water molecules break apart the solid. What Are Ionic Compounds Known As When Dissolved In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Are Ionic Compounds Known As When Dissolved In Water Find out which compounds are. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. For example, sodium chloride (nacl).. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download What Are Ionic Compounds Known As When Dissolved In Water learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. when ionic compounds dissolve in water, the ions in the solid separate. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. Find out which compounds are. learn how ionic compounds dissociate and disperse in water to form electrolytes. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. when ionic compounds dissolve in water,. What Are Ionic Compounds Known As When Dissolved In Water.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps What Are Ionic Compounds Known As When Dissolved In Water learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. For example, sodium chloride (nacl). study with quizlet and memorize flashcards containing. What Are Ionic Compounds Known As When Dissolved In Water.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry What Are Ionic Compounds Known As When Dissolved In Water For example, sodium chloride (nacl). learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Find out which compounds are. study. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT Ionic Compounds Names and Formulas PowerPoint Presentation, free What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. Find out which compounds are. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. For example, sodium chloride (nacl). learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic. What Are Ionic Compounds Known As When Dissolved In Water.
From testbook.com
Formation Of Ionic Compounds Learn Definition, Examples & Uses What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. study with quizlet and memorize flashcards containing terms like. What Are Ionic Compounds Known As When Dissolved In Water.
From brentongokemckay.blogspot.com
What Happens When an Ionic Compound Dissolves in Water What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Find out which compounds are. . What Are Ionic Compounds Known As When Dissolved In Water.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination What Are Ionic Compounds Known As When Dissolved In Water Find out which compounds are. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. . What Are Ionic Compounds Known As When Dissolved In Water.
From dxomdlczn.blob.core.windows.net
Are Ionic Compounds Liquid at Anthony Herring blog What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. learn how ionic compounds dissociate and disperse in water to form electrolytes. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. For example, sodium chloride (nacl). study with quizlet and. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. learn how ionic compounds dissociate and disperse in water to form electrolytes. For example, sodium chloride (nacl). when ionic compounds dissolve in water, the. What Are Ionic Compounds Known As When Dissolved In Water.
From www.learnatnoon.com
Physical Properties of an Ionic Compound Noon Academy What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. What Are Ionic Compounds Known As When Dissolved In Water.
From studylib.net
Naming Ionic Compounds What Are Ionic Compounds Known As When Dissolved In Water Find out which compounds are. when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. learn how ionic compounds dissociate and disperse in water to form electrolytes. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. For example, sodium chloride (nacl). study. What Are Ionic Compounds Known As When Dissolved In Water.
From socratic.org
What is the chemical equation for HCl dissolving into water and What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. learn how ionic compounds dissociate and disperse in water to form electrolytes. when ionic compounds dissolve in water, the. What Are Ionic Compounds Known As When Dissolved In Water.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision What Are Ionic Compounds Known As When Dissolved In Water study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. Find out which compounds are. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. study with quizlet and memorize flashcards containing terms like when soluble ionic compounds are placed in water, their ions. For. What Are Ionic Compounds Known As When Dissolved In Water.
From www.slideserve.com
PPT IONIC COMPOUNDS PowerPoint Presentation, free download ID2435238 What Are Ionic Compounds Known As When Dissolved In Water learn how ionic compounds dissociate and disperse in water to form electrolytes. For example, sodium chloride (nacl). when ionic compounds are dissolved into water, the polar water molecules break apart the solid crystal. study with quizlet and memorize flashcards containing terms like what determines whether an ionic compound will dissolve. when ionic compounds dissolve in water,. What Are Ionic Compounds Known As When Dissolved In Water.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? What Are Ionic Compounds Known As When Dissolved In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. For example, sodium chloride (nacl). when. What Are Ionic Compounds Known As When Dissolved In Water.