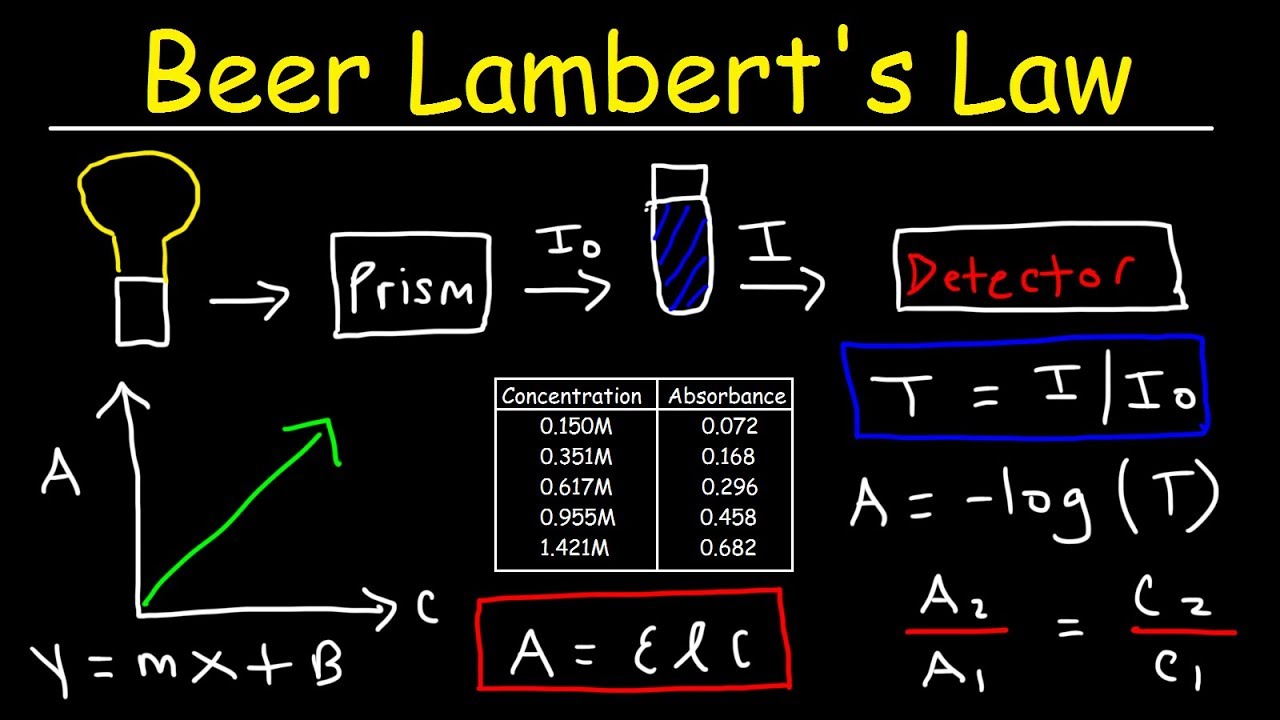
Beer Lambert Law Absorbance Units . Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. You can also use this calculator to.
from www.youtube.com
Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. The absorbance (a) is a unitless number because io i i.
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry
Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Absorbance Units You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. Beer Lambert Law Absorbance Units.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Beer Lambert Law Absorbance Units.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From cevhwrun.blob.core.windows.net
Beer Lambert Law Relationship Between Absorbance And Concentration at Beer Lambert Law Absorbance Units You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. Beer Lambert Law Absorbance Units.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Beer Lambert Law Absorbance Units.
From www.slideserve.com
PPT Determining An Equilibrium Constant Using Spectrophotometry and Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From ceajxuqm.blob.core.windows.net
Beer Lambert Law Frq at Aaron Salinas blog Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From exodtohyt.blob.core.windows.net
Beer Lambert Law Scientific Journal at Darrell Dewitt blog Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From joimeihka.blob.core.windows.net
Beer Lambert Law Linear Equation at Kimberly Graves blog Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.chegg.com
Solved BeerLambert law states the absorbance of a solution Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From public.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From joibnsohl.blob.core.windows.net
Spectrophotometry Learn The BeerLambert Law With Absorbance Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.numerade.com
SOLVED Write down an expression for the BeerLambert Law in terms of Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Beer Lambert Law Absorbance Units.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.scribd.com
Beer Lambert Law Absorbance Mole (Unit) Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.researchgate.net
Measuring transmittance and absorbance using BeerLambert's law Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.researchgate.net
(a) Calculated absorbance unit by BeerLambert Law for A. hydrophila Beer Lambert Law Absorbance Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.numerade.com
SOLVED 10 What is the unit of absorbance which can be X ?derived from Beer Lambert Law Absorbance Units You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. Beer Lambert Law Absorbance Units.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From stock.adobe.com
Vector scheme of Beer Lambert law. Cuvette with the blue liquid sample Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Absorbance Units You can also use this calculator to. The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Absorbance Units.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Absorbance Units The absorbance (a) is a unitless number because io i i. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law Absorbance Units.
From www.slideserve.com
PPT Absorption and Scattering PowerPoint Presentation, free download Beer Lambert Law Absorbance Units You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The absorbance (a) is a unitless number because io i i. Beer Lambert Law Absorbance Units.