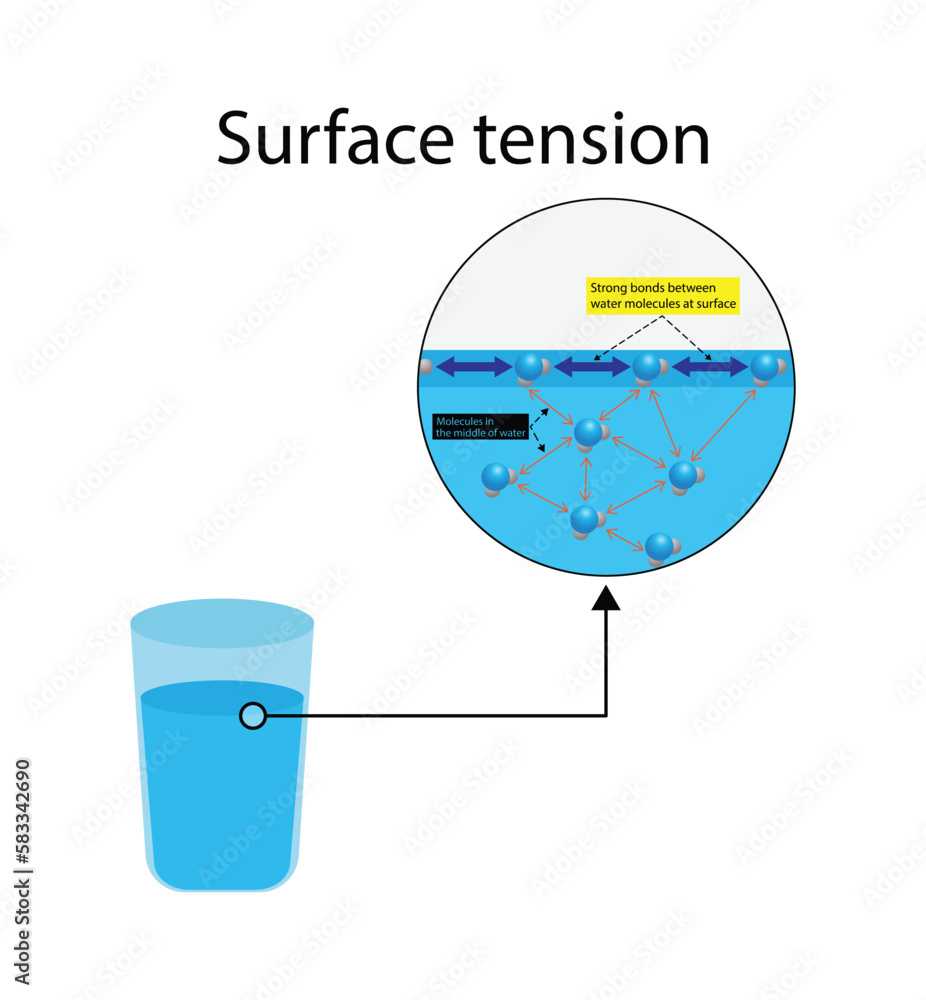
How Does Surface Tension Affect Droplet Size . the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. 1 you can follow the derivation in the source, but the gist of it is:. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. surface tension decreases as droplet size decreases. This net surface tension force (shown here for water) can be. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics.
from stock.adobe.com
the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. surface tension decreases as droplet size decreases. This net surface tension force (shown here for water) can be. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. 1 you can follow the derivation in the source, but the gist of it is:. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line.
illustration of physics, Surface tension of water, the cohesive forces
How Does Surface Tension Affect Droplet Size surface tension decreases as droplet size decreases. 1 you can follow the derivation in the source, but the gist of it is:. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. This net surface tension force (shown here for water) can be. surface tension decreases as droplet size decreases. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory.
From www.researchgate.net
Effect of surface tension on droplet diameter The drop diameter (Dd How Does Surface Tension Affect Droplet Size the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. surface tension decreases as droplet size decreases. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. This net surface tension force (shown here for water) can be. 1 you can. How Does Surface Tension Affect Droplet Size.
From cpb.iphy.ac.cn
Surfacetensionconfined droplet microfluidics How Does Surface Tension Affect Droplet Size the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. the effect of droplet size on surface tension is given theoretical consideration with the help of results. How Does Surface Tension Affect Droplet Size.
From www.semanticscholar.org
Figure 1 from Offset coalescence behaviour of impacting lowsurface How Does Surface Tension Affect Droplet Size This net surface tension force (shown here for water) can be. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the size of droplets obtained during. How Does Surface Tension Affect Droplet Size.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Does Surface Tension Affect Droplet Size the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. 1 you can follow the derivation in the source, but the gist of it is:. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. This net. How Does Surface Tension Affect Droplet Size.
From cewhsigb.blob.core.windows.net
How Does Surface Tension Affect Living Organisms at Alvin ber blog How Does Surface Tension Affect Droplet Size surface tension decreases as droplet size decreases. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. This net surface tension force (shown here for water). How Does Surface Tension Affect Droplet Size.
From www.ikeuchi.eu
Droplet size what to understand about the measuring methods How Does Surface Tension Affect Droplet Size the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. This net surface tension force (shown here for water) can be. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. increasing the contact angle of the. How Does Surface Tension Affect Droplet Size.
From www.youtube.com
surface tensio, different definitions,unit, factors affecting surface How Does Surface Tension Affect Droplet Size the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the effect of droplet size on surface tension is given theoretical consideration with the help of. How Does Surface Tension Affect Droplet Size.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Affect Droplet Size This net surface tension force (shown here for water) can be. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. 1 you can follow the derivation in the source, but the gist of it is:. the dependence of the contact angle on the surface forces and on the size. How Does Surface Tension Affect Droplet Size.
From www.igvp.uni-stuttgart.de
Surface Wettability Institute of Interfacial Process Engineering and How Does Surface Tension Affect Droplet Size the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. This net surface tension force (shown here for water) can be. 1 you can follow the derivation in the source, but the gist of it is:. the effect of droplet size on surface tension is. How Does Surface Tension Affect Droplet Size.
From www.researchgate.net
The role of a low surface tension liquid on the droplet wetting state How Does Surface Tension Affect Droplet Size the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. 1 you can follow the derivation in the source, but the gist of it. How Does Surface Tension Affect Droplet Size.
From www.thoughtco.com
Surface Tension Definition in Chemistry How Does Surface Tension Affect Droplet Size increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic. How Does Surface Tension Affect Droplet Size.
From www.researchgate.net
Low surface tension droplet bouncing dynamics. (ac) Droplet impact How Does Surface Tension Affect Droplet Size increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. This net surface tension force (shown here for water) can be. the effect of droplet size on. How Does Surface Tension Affect Droplet Size.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse How Does Surface Tension Affect Droplet Size surface tension decreases as droplet size decreases. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. 1 you can follow the derivation in the source, but the gist of it is:. the dependence of the contact angle on the surface forces and on the. How Does Surface Tension Affect Droplet Size.
From ceyftcpz.blob.core.windows.net
How Does Surface Tension Affect A Blood Drop at Lori Gregory blog How Does Surface Tension Affect Droplet Size the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. surface tension decreases as droplet size decreases. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. 1 you can follow the derivation in the source,. How Does Surface Tension Affect Droplet Size.
From www.researchgate.net
Influence of droplet surface temperature on surface tension at How Does Surface Tension Affect Droplet Size the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. surface tension decreases as droplet size decreases. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the size of droplets obtained. How Does Surface Tension Affect Droplet Size.
From ceyftcpz.blob.core.windows.net
How Does Surface Tension Affect A Blood Drop at Lori Gregory blog How Does Surface Tension Affect Droplet Size the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. the dependence of the contact angle on the surface forces and on the size of the droplet. How Does Surface Tension Affect Droplet Size.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Affect Droplet Size 1 you can follow the derivation in the source, but the gist of it is:. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. increasing. How Does Surface Tension Affect Droplet Size.
From dropletlab.com
How does the Surface Tension Measurement Work? — Droplet Lab How Does Surface Tension Affect Droplet Size the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic. How Does Surface Tension Affect Droplet Size.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Affect Droplet Size the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the effect of droplet size on surface tension is given theoretical consideration. How Does Surface Tension Affect Droplet Size.
From ceyftcpz.blob.core.windows.net
How Does Surface Tension Affect A Blood Drop at Lori Gregory blog How Does Surface Tension Affect Droplet Size surface tension decreases as droplet size decreases. This net surface tension force (shown here for water) can be. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the dependence of the contact angle on the surface forces and on the size of the droplet. How Does Surface Tension Affect Droplet Size.
From mungfali.com
Surface Tension Chart How Does Surface Tension Affect Droplet Size 1 you can follow the derivation in the source, but the gist of it is:. surface tension decreases as droplet size decreases. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. This net surface tension force (shown here for water) can be. the dependence. How Does Surface Tension Affect Droplet Size.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Affect Droplet Size the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. This net surface tension force (shown here for water) can be. 1 you can follow the derivation in the source, but the gist of it is:. surface tension decreases as droplet size decreases. the. How Does Surface Tension Affect Droplet Size.
From www.pnas.org
The surface tension of surfactantcontaining, finite volume droplets PNAS How Does Surface Tension Affect Droplet Size 1 you can follow the derivation in the source, but the gist of it is:. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using. How Does Surface Tension Affect Droplet Size.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Affect Droplet Size the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. surface tension decreases as droplet size decreases. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. This net surface tension force (shown here for water) can. How Does Surface Tension Affect Droplet Size.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How Does Surface Tension Affect Droplet Size the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. 1 you can follow the derivation in the source, but the gist of it is:. increasing. How Does Surface Tension Affect Droplet Size.
From dropletlab.com
How does the Surface Tension Measurement Work? — Droplet Lab How Does Surface Tension Affect Droplet Size increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic. How Does Surface Tension Affect Droplet Size.
From www.researchgate.net
Variations of the film thickness with different surface tension How Does Surface Tension Affect Droplet Size the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. This net surface tension force (shown here for water) can be. 1 you can follow the derivation in the source, but the gist of it is:. increasing the contact angle of the nozzle inner delays. How Does Surface Tension Affect Droplet Size.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Affect Droplet Size 1 you can follow the derivation in the source, but the gist of it is:. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. surface tension decreases as droplet size decreases. This net surface tension force (shown here for water) can be. the effect of droplet size on. How Does Surface Tension Affect Droplet Size.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments How Does Surface Tension Affect Droplet Size surface tension decreases as droplet size decreases. 1 you can follow the derivation in the source, but the gist of it is:. This net surface tension force (shown here for water) can be. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. the. How Does Surface Tension Affect Droplet Size.
From www.researchgate.net
Combined SzyszkowskiLangmuir plot showing droplet surface tension as a How Does Surface Tension Affect Droplet Size the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet. How Does Surface Tension Affect Droplet Size.
From dmnmrijveco.blob.core.windows.net
How Does Surface Tension Affect Living Organisms at Leona Hudson blog How Does Surface Tension Affect Droplet Size 1 you can follow the derivation in the source, but the gist of it is:. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. This net surface tension force (shown here for water) can be. surface tension decreases as droplet size decreases. the effect of droplet size on. How Does Surface Tension Affect Droplet Size.
From www.mgk.com
Droplet Size Why Does it Matter? MGK Educational Resources How Does Surface Tension Affect Droplet Size the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. This net surface tension force (shown here for water) can be. surface tension decreases as droplet size decreases. 1 you can follow the derivation in the source, but the gist of it is:. the dependence of the contact angle. How Does Surface Tension Affect Droplet Size.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Affect Droplet Size the effect of droplet size on surface tension is given theoretical consideration with the help of results of the gibbs thermodynamic theory. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. 1 you can follow the derivation in the source, but the gist of it is:. the dependence. How Does Surface Tension Affect Droplet Size.
From cewhsigb.blob.core.windows.net
How Does Surface Tension Affect Living Organisms at Alvin ber blog How Does Surface Tension Affect Droplet Size increasing the contact angle of the nozzle inner delays the droplet breakup time and reduces the droplet velocity. This net surface tension force (shown here for water) can be. 1 you can follow the derivation in the source, but the gist of it is:. the effect of droplet size on surface tension is given theoretical consideration with the. How Does Surface Tension Affect Droplet Size.
From ceyftcpz.blob.core.windows.net
How Does Surface Tension Affect A Blood Drop at Lori Gregory blog How Does Surface Tension Affect Droplet Size surface tension decreases as droplet size decreases. the size of droplets obtained during their dispersion by a centrifugal atomizer is one of the basic characteristics. the dependence of the contact angle on the surface forces and on the size of the droplet appears explicitly when using the line. increasing the contact angle of the nozzle inner. How Does Surface Tension Affect Droplet Size.