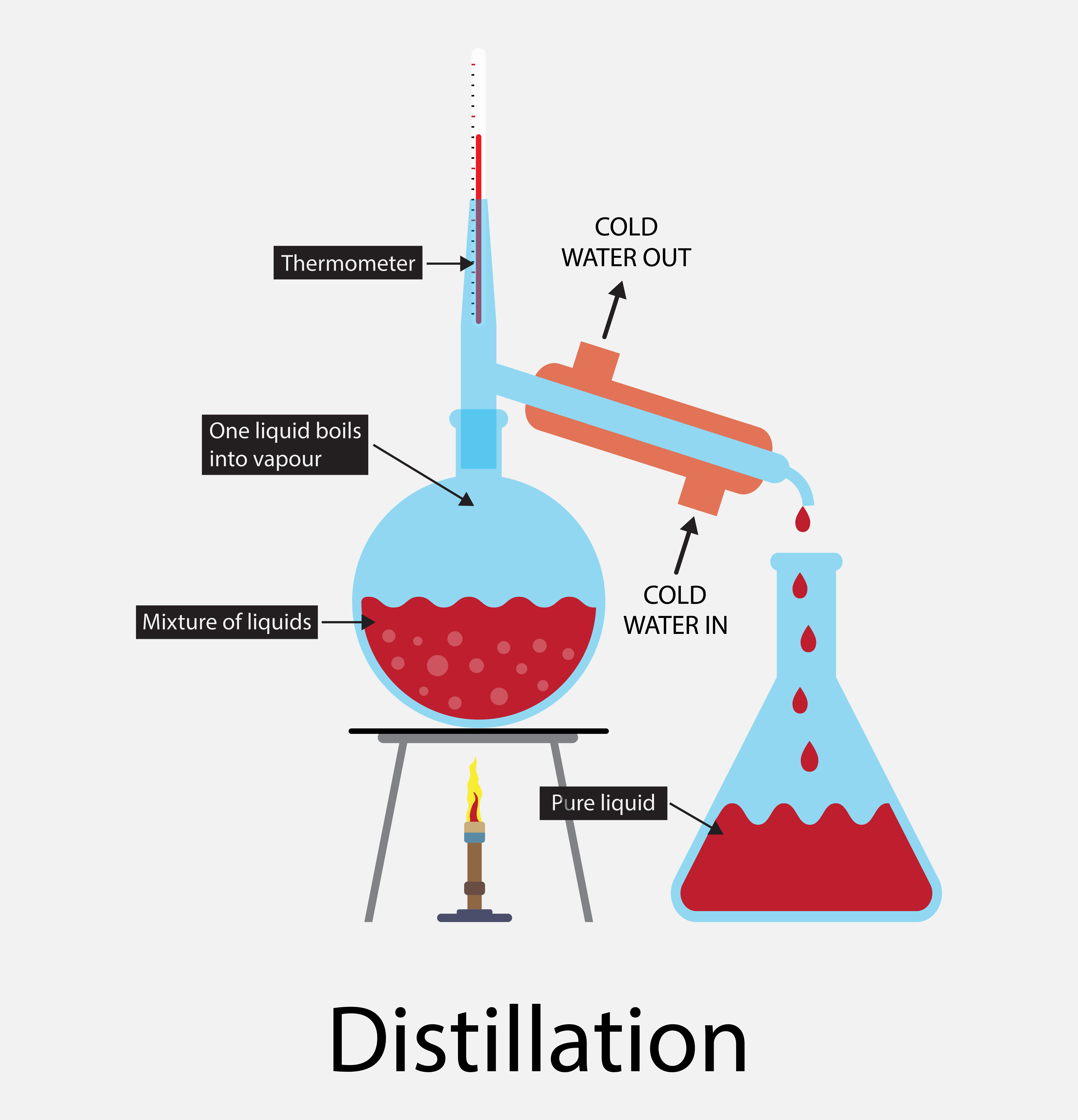
Can Sugar And Water Be Separated By Distillation . After collecting the distillate, you. Heat the mixture in a flask until the gas above the. A mixture of two liquids can be separated by either distillation method. However, simple distillation requires very careful control of the. For example, this method can separate a homogeneous mixture of sugar and water. By distillation, the alcohol can be separated from the fruit juice. The correct option is b distillation. Take the solution of sugar and water in. Fruit juices contain both water and sugar, which have different boiling points. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. This method works because the. Simple distillation can be used to separate sugar and water from sugar solution. Water and ethanol can be separated using distillation by following these steps: If you wanted to isolate pure solvent from the solution, you would need to use distillation.
from primaryleap.co.uk
Water and ethanol can be separated using distillation by following these steps: Fruit juices contain both water and sugar, which have different boiling points. However, simple distillation requires very careful control of the. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. After collecting the distillate, you. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. The correct option is b distillation. If you wanted to isolate pure solvent from the solution, you would need to use distillation. This method works because the. A mixture of two liquids can be separated by either distillation method.
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap
Can Sugar And Water Be Separated By Distillation If you wanted to isolate pure solvent from the solution, you would need to use distillation. For example, this method can separate a homogeneous mixture of sugar and water. Simple distillation can be used to separate sugar and water from sugar solution. This method works because the. Heat the mixture in a flask until the gas above the. By distillation, the alcohol can be separated from the fruit juice. A mixture of two liquids can be separated by either distillation method. However, simple distillation requires very careful control of the. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Water and ethanol can be separated using distillation by following these steps: Take the solution of sugar and water in. If you wanted to isolate pure solvent from the solution, you would need to use distillation. Fruit juices contain both water and sugar, which have different boiling points. After collecting the distillate, you. The correct option is b distillation.
From nathalie-bogspotsimpson.blogspot.com
Can Sugar Be Separated From Water Can Sugar And Water Be Separated By Distillation For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. The correct option is b distillation. Fruit juices contain both water and sugar, which have different boiling points. For example, this method can separate a homogeneous mixture of sugar and water. Take the solution of sugar and water in. By distillation, the alcohol. Can Sugar And Water Be Separated By Distillation.
From www.studypage.in
Separation of Mixtures Study Page Can Sugar And Water Be Separated By Distillation After collecting the distillate, you. If you wanted to isolate pure solvent from the solution, you would need to use distillation. However, simple distillation requires very careful control of the. By distillation, the alcohol can be separated from the fruit juice. Take the solution of sugar and water in. Water and ethanol can be separated using distillation by following these. Can Sugar And Water Be Separated By Distillation.
From stock.adobe.com
Separation of mixtures. Basic Distillation. You can do simple Can Sugar And Water Be Separated By Distillation For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. However, simple distillation requires very careful control of the. By distillation, the alcohol can be separated from the fruit juice. Water and ethanol can be separated using distillation by following these steps: Simple distillation can be used to separate sugar and water from. Can Sugar And Water Be Separated By Distillation.
From byjus.com
2. How to separate sugar and water solution? Can Sugar And Water Be Separated By Distillation For example, this method can separate a homogeneous mixture of sugar and water. Heat the mixture in a flask until the gas above the. Fruit juices contain both water and sugar, which have different boiling points. If you wanted to isolate pure solvent from the solution, you would need to use distillation. However, simple distillation requires very careful control of. Can Sugar And Water Be Separated By Distillation.
From www.geeksforgeeks.org
Separation of Mixtures Can Sugar And Water Be Separated By Distillation This method works because the. A mixture of two liquids can be separated by either distillation method. After collecting the distillate, you. Take the solution of sugar and water in. Simple distillation can be used to separate sugar and water from sugar solution. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling. Can Sugar And Water Be Separated By Distillation.
From www.sliderbase.com
The Organization of Matter Presentation Chemistry Can Sugar And Water Be Separated By Distillation This method works because the. A mixture of two liquids can be separated by either distillation method. If you wanted to isolate pure solvent from the solution, you would need to use distillation. Take the solution of sugar and water in. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. The correct. Can Sugar And Water Be Separated By Distillation.
From www.geeksforgeeks.org
Separation by Distillation Can Sugar And Water Be Separated By Distillation Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. Heat the mixture in a flask until the gas above the. If you wanted to isolate pure solvent from the solution, you would need to use distillation. Fruit juices contain both water and sugar, which have different boiling points. For example, this. Can Sugar And Water Be Separated By Distillation.
From www.britannica.com
distillation summary Britannica Can Sugar And Water Be Separated By Distillation This method works because the. A mixture of two liquids can be separated by either distillation method. Fruit juices contain both water and sugar, which have different boiling points. The correct option is b distillation. For example, this method can separate a homogeneous mixture of sugar and water. If you wanted to isolate pure solvent from the solution, you would. Can Sugar And Water Be Separated By Distillation.
From slidetodoc.com
Properties of matter Pure Substances Mixtures Solutions Separation Can Sugar And Water Be Separated By Distillation Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. For example, this method can separate a homogeneous mixture of sugar and water. Fruit juices contain both water and sugar, which have different boiling points. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation.. Can Sugar And Water Be Separated By Distillation.
From classnotes.org.in
Evaporation, Distillation Class 6, Separation of Substances Can Sugar And Water Be Separated By Distillation For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. The correct option is b distillation. By distillation, the alcohol can be separated from the fruit juice. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. Heat the mixture in a flask until the. Can Sugar And Water Be Separated By Distillation.
From labeled-diagram.blogspot.com
Draw A Neat Labelled Diagram Of Simple Distillation Process Labeled Can Sugar And Water Be Separated By Distillation If you wanted to isolate pure solvent from the solution, you would need to use distillation. Fruit juices contain both water and sugar, which have different boiling points. A mixture of two liquids can be separated by either distillation method. However, simple distillation requires very careful control of the. This method works because the. Take the solution of sugar and. Can Sugar And Water Be Separated By Distillation.
From www.slideserve.com
PPT Separating Mixtures PowerPoint Presentation, free download ID Can Sugar And Water Be Separated By Distillation Heat the mixture in a flask until the gas above the. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. Water and ethanol can be separated using distillation by following these steps: Fruit juices contain both water and sugar, which have different boiling points. For example, liquid ethanol can be separated. Can Sugar And Water Be Separated By Distillation.
From www.ck12.org
Separating Mixtures CK12 Foundation Can Sugar And Water Be Separated By Distillation Take the solution of sugar and water in. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. Water and ethanol can be separated using distillation by following these steps: This method works because the. The correct option is b distillation. Fruit juices contain both water and sugar, which have different boiling. Can Sugar And Water Be Separated By Distillation.
From stock.adobe.com
Vecteur Stock Chemical illustration. The solubility of sugar and water Can Sugar And Water Be Separated By Distillation Take the solution of sugar and water in. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Heat the mixture in a flask until the gas above the. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. After collecting the distillate, you. Water. Can Sugar And Water Be Separated By Distillation.
From issuu.com
How To Separate Sugar And Water Chemistry? by AmericanLifeguardManual Can Sugar And Water Be Separated By Distillation Heat the mixture in a flask until the gas above the. If you wanted to isolate pure solvent from the solution, you would need to use distillation. The correct option is b distillation. Simple distillation can be used to separate sugar and water from sugar solution. Fruit juices contain both water and sugar, which have different boiling points. After collecting. Can Sugar And Water Be Separated By Distillation.
From primaryleap.co.uk
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap Can Sugar And Water Be Separated By Distillation Take the solution of sugar and water in. The correct option is b distillation. Water and ethanol can be separated using distillation by following these steps: A mixture of two liquids can be separated by either distillation method. However, simple distillation requires very careful control of the. After collecting the distillate, you. Heat the mixture in a flask until the. Can Sugar And Water Be Separated By Distillation.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Can Sugar And Water Be Separated By Distillation Heat the mixture in a flask until the gas above the. Simple distillation can be used to separate sugar and water from sugar solution. After collecting the distillate, you. However, simple distillation requires very careful control of the. Take the solution of sugar and water in. By distillation, the alcohol can be separated from the fruit juice. The correct option. Can Sugar And Water Be Separated By Distillation.
From www.youtube.com
Separating Liquids by Distillation YouTube Can Sugar And Water Be Separated By Distillation If you wanted to isolate pure solvent from the solution, you would need to use distillation. Take the solution of sugar and water in. After collecting the distillate, you. Simple distillation can be used to separate sugar and water from sugar solution. Fruit juices contain both water and sugar, which have different boiling points. For example, this method can separate. Can Sugar And Water Be Separated By Distillation.
From www.slideserve.com
PPT To learn to distinguish between mixtures and pure substances To Can Sugar And Water Be Separated By Distillation Fruit juices contain both water and sugar, which have different boiling points. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. By distillation, the alcohol can be separated from the fruit juice. This method works because the. However, simple distillation requires very careful control of the. Water and ethanol can be. Can Sugar And Water Be Separated By Distillation.
From www.shalom-education.com
Simple and Fractional Distillation KS3 Chemistry Revision Can Sugar And Water Be Separated By Distillation Water and ethanol can be separated using distillation by following these steps: For example, this method can separate a homogeneous mixture of sugar and water. Take the solution of sugar and water in. After collecting the distillate, you. Simple distillation can be used to separate sugar and water from sugar solution. By distillation, the alcohol can be separated from the. Can Sugar And Water Be Separated By Distillation.
From www.dreamstime.com
Distillation Vector Illustration. Liquid Substance Separation Can Sugar And Water Be Separated By Distillation Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. This method works because the. If you wanted to isolate pure solvent from the solution, you would need to use distillation. However, simple distillation requires very careful control of the. Fruit juices contain both water and sugar, which have different boiling points.. Can Sugar And Water Be Separated By Distillation.
From stock.adobe.com
Video „Basic distillation or dropping. Separating mixtures. You can do Can Sugar And Water Be Separated By Distillation For example, this method can separate a homogeneous mixture of sugar and water. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. The correct option is b distillation. If you wanted to isolate pure solvent from the solution, you would need to use distillation. Simple distillation can be used to separate sugar. Can Sugar And Water Be Separated By Distillation.
From stacklima.com
Séparation par distillation fractionnée StackLima Can Sugar And Water Be Separated By Distillation Water and ethanol can be separated using distillation by following these steps: Fruit juices contain both water and sugar, which have different boiling points. The correct option is b distillation. However, simple distillation requires very careful control of the. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. For example, liquid. Can Sugar And Water Be Separated By Distillation.
From kunduz.com
Heterogeneous Mixtures Definition, Properties, Types, Examples Can Sugar And Water Be Separated By Distillation Fruit juices contain both water and sugar, which have different boiling points. By distillation, the alcohol can be separated from the fruit juice. For example, this method can separate a homogeneous mixture of sugar and water. The correct option is b distillation. Simple distillation can be used to separate sugar and water from sugar solution. Water and ethanol can be. Can Sugar And Water Be Separated By Distillation.
From www.vectorstock.com
Diagram showing distillation separating mixtures Vector Image Can Sugar And Water Be Separated By Distillation After collecting the distillate, you. This method works because the. Simple distillation can be used to separate sugar and water from sugar solution. However, simple distillation requires very careful control of the. A mixture of two liquids can be separated by either distillation method. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their. Can Sugar And Water Be Separated By Distillation.
From primaryleap.co.uk
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap Can Sugar And Water Be Separated By Distillation Fruit juices contain both water and sugar, which have different boiling points. If you wanted to isolate pure solvent from the solution, you would need to use distillation. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. However, simple distillation requires very careful control of the. Take the solution of sugar and. Can Sugar And Water Be Separated By Distillation.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Can Sugar And Water Be Separated By Distillation By distillation, the alcohol can be separated from the fruit juice. If you wanted to isolate pure solvent from the solution, you would need to use distillation. This method works because the. However, simple distillation requires very careful control of the. Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. Take. Can Sugar And Water Be Separated By Distillation.
From studylib.net
Experiment 2. Separation of Liquids by Distillation. Can Sugar And Water Be Separated By Distillation Water and ethanol can be separated using distillation by following these steps: However, simple distillation requires very careful control of the. After collecting the distillate, you. If you wanted to isolate pure solvent from the solution, you would need to use distillation. The correct option is b distillation. Heat the mixture in a flask until the gas above the. Take. Can Sugar And Water Be Separated By Distillation.
From www.slideshare.net
Extraction theory Can Sugar And Water Be Separated By Distillation Heat the mixture in a flask until the gas above the. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Fruit juices contain both water and sugar, which have different boiling points. The correct option is b distillation. A mixture of two liquids can be separated by either distillation method. Water and. Can Sugar And Water Be Separated By Distillation.
From www.slideserve.com
PPT Methods of Separating Mixtures PowerPoint Presentation, free Can Sugar And Water Be Separated By Distillation If you wanted to isolate pure solvent from the solution, you would need to use distillation. However, simple distillation requires very careful control of the. Simple distillation can be used to separate sugar and water from sugar solution. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. By distillation, the alcohol can. Can Sugar And Water Be Separated By Distillation.
From www.bbc.co.uk
Distillation BBC Bitesize Can Sugar And Water Be Separated By Distillation Petroleum is a mixture of many different hydrocarbons, and distillation can separate them based on their boiling points. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Simple distillation can be used to separate sugar and water from sugar solution. By distillation, the alcohol can be separated from the fruit juice. After. Can Sugar And Water Be Separated By Distillation.
From www.slideserve.com
PPT Separation Techniques Grade 10 Chemistry PowerPoint Presentation Can Sugar And Water Be Separated By Distillation For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Water and ethanol can be separated using distillation by following these steps: Heat the mixture in a flask until the gas above the. By distillation, the alcohol can be separated from the fruit juice. If you wanted to isolate pure solvent from the. Can Sugar And Water Be Separated By Distillation.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3333021 Can Sugar And Water Be Separated By Distillation A mixture of two liquids can be separated by either distillation method. Water and ethanol can be separated using distillation by following these steps: For example, this method can separate a homogeneous mixture of sugar and water. If you wanted to isolate pure solvent from the solution, you would need to use distillation. This method works because the. The correct. Can Sugar And Water Be Separated By Distillation.
From www.bbc.co.uk
Distillation BBC Bitesize Can Sugar And Water Be Separated By Distillation Water and ethanol can be separated using distillation by following these steps: The correct option is b distillation. By distillation, the alcohol can be separated from the fruit juice. Simple distillation can be used to separate sugar and water from sugar solution. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Petroleum. Can Sugar And Water Be Separated By Distillation.
From www.pinterest.co.uk
Distillation Process Science HowThingsWork Gif Distillation Can Sugar And Water Be Separated By Distillation However, simple distillation requires very careful control of the. A mixture of two liquids can be separated by either distillation method. The correct option is b distillation. For example, this method can separate a homogeneous mixture of sugar and water. Take the solution of sugar and water in. Simple distillation can be used to separate sugar and water from sugar. Can Sugar And Water Be Separated By Distillation.