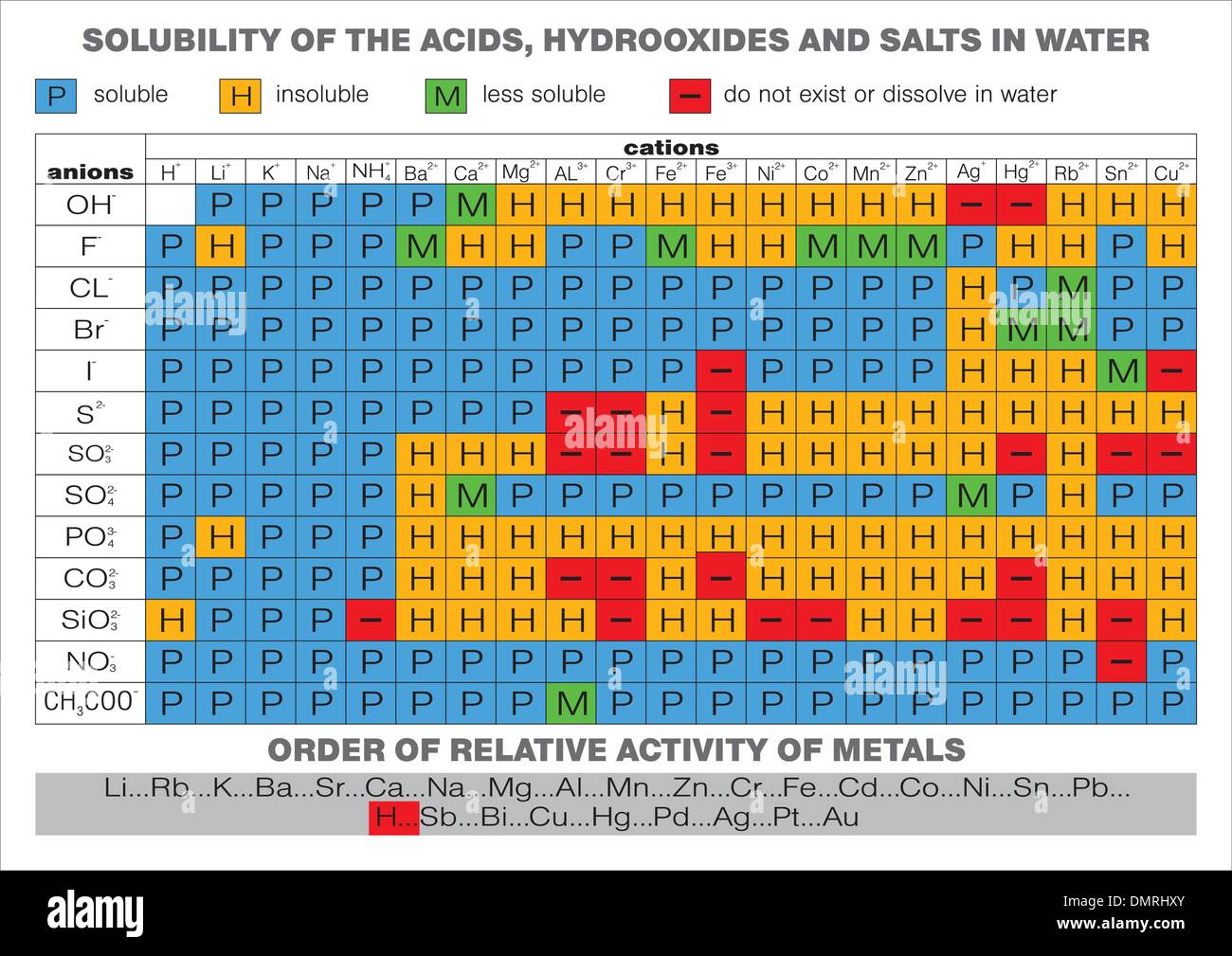
List Of Hydroxides . The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. Oh − is a diatomic anion with the chemical name hydroxide. This list may not reflect recent changes. The following 95 pages are in this category, out of 95 total. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins.
from www.alamy.com
Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This list may not reflect recent changes. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. Oh − is a diatomic anion with the chemical name hydroxide. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The following 95 pages are in this category, out of 95 total.
Hydroxides Stock Vector Images Alamy
List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. This list may not reflect recent changes. The following 95 pages are in this category, out of 95 total. Oh − is a diatomic anion with the chemical name hydroxide. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion.
From pediaa.com
Difference Between Alkali and Metal Hydroxide Definition, Formation List Of Hydroxides Oh − is a diatomic anion with the chemical name hydroxide. This list may not reflect recent changes. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases.. List Of Hydroxides.
From www.alamy.com
Hydroxides Stock Vector Images Alamy List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. Oh − is a diatomic anion with the chemical name hydroxide. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. This list may not reflect recent changes.. List Of Hydroxides.
From www.slideserve.com
PPT CHAPTER 19 PowerPoint Presentation, free download ID3515833 List Of Hydroxides All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. Oh − is a diatomic anion with the chemical name hydroxide. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered. List Of Hydroxides.
From www.slideserve.com
PPT The sBlock Elements PowerPoint Presentation, free download ID List Of Hydroxides This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. This list may not reflect recent changes. The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. All hydroxides form at low temperatures and are found predominantly as. List Of Hydroxides.
From www.onlinechemistrytutor.net
metalhydroxidetestscopy001 Online Chemistry Tutor List Of Hydroxides The following 95 pages are in this category, out of 95 total. This list may not reflect recent changes. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong. List Of Hydroxides.
From www.chegg.com
Solved Flow Chart 2 The Hydroxides List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. This list may not reflect recent changes. The following 95 pages are in this category, out of 95 total. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. All hydroxides form at low temperatures. List Of Hydroxides.
From www.slideserve.com
PPT Solubility Rules PowerPoint Presentation, free download ID4566194 List Of Hydroxides Oh − is a diatomic anion with the chemical name hydroxide. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong. List Of Hydroxides.
From www.slideserve.com
PPT Group 2 Elements & Compounds PowerPoint Presentation, free List Of Hydroxides This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. Oh − is a diatomic anion with the chemical name hydroxide. The following 95 pages are in this category, out of 95 total. This list may not reflect recent changes. All hydroxides form at low temperatures and are found predominantly as weathering. List Of Hydroxides.
From saylordotorg.github.io
Solubility and pH List Of Hydroxides This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The following 95 pages are in this category, out of 95 total. Oh − is a diatomic anion with the chemical. List Of Hydroxides.
From www.slideserve.com
PPT Solubility of metal hydroxides, and amphoteric behavior List Of Hydroxides This list may not reflect recent changes. The following 95 pages are in this category, out of 95 total. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. Oh − is a diatomic anion with the chemical name hydroxide. The hydroxides of the alkali metals, lithium, sodium, potassium,. List Of Hydroxides.
From www.slideserve.com
PPT The sBlock Elements PowerPoint Presentation, free download ID List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. This list may not reflect recent changes. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. Hydroxide is also called hydroxyl or hydroxyl radical. List Of Hydroxides.
From www.researchgate.net
Solid metal hydroxides, oxidehydroxides, oxides, and ionic species List Of Hydroxides This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. Oh − is a diatomic anion with the chemical name hydroxide. This list may not reflect recent changes. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. All hydroxides form. List Of Hydroxides.
From kidspressmagazine.com
Acids and Bases List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. Oh − is a diatomic anion with the chemical name hydroxide. The following 95 pages are in this category, out of 95 total. The hydroxides of the group i (alkali metals) and group ii (alkaline earth). List Of Hydroxides.
From www.slideserve.com
PPT Solubility of metal hydroxides, and amphoteric behavior List Of Hydroxides All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. Oh − is a diatomic anion with the chemical name hydroxide. The hydroxides of the group i (alkali metals) and group. List Of Hydroxides.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记2.2.3 Group 2 Hydroxides & Sulfates翰林国际教育 List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. This list may not reflect recent changes. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to. List Of Hydroxides.
From www.researchgate.net
Schematic of intercalation compounds prepared by solidsolid reactions List Of Hydroxides All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The following 95 pages are in this category, out of 95 total. Oh − is a diatomic anion. List Of Hydroxides.
From www.researchgate.net
Constants of Solubility Products of Metal Hydroxides Download Table List Of Hydroxides The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,.. List Of Hydroxides.
From mmerevise.co.uk
Group 2 Solubility and Chemical Tests Revision MME List Of Hydroxides Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. This list may not reflect recent changes. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The hydroxides of. List Of Hydroxides.
From slideplayer.com
Chapter 15 Acids and Bases ppt download List Of Hydroxides The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The following 95 pages are in this category, out of 95 total. The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. Oh − is. List Of Hydroxides.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 2.7.3 Reactions of Acids翰林国际教育 List Of Hydroxides The following 95 pages are in this category, out of 95 total. This list may not reflect recent changes. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The hydroxides of the group i (alkali metals) and group ii (alkaline. List Of Hydroxides.
From brainly.in
Name all groups like hydroxide group,sulphate group,nitrate group, and List Of Hydroxides This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The following 95 pages are in this category, out of 95 total. This list may not reflect recent changes. The hydroxides. List Of Hydroxides.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID List Of Hydroxides Oh − is a diatomic anion with the chemical name hydroxide. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium,. List Of Hydroxides.
From www.slideserve.com
PPT Oxides and Hydroxides PowerPoint Presentation, free download ID List Of Hydroxides This list may not reflect recent changes. Oh − is a diatomic anion with the chemical name hydroxide. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. The following 95 pages are in this category, out of 95 total. Hydroxide is also called hydroxyl or hydroxyl radical or. List Of Hydroxides.
From www.slideserve.com
PPT chemical nomenclature (textbook 12 25) PowerPoint List Of Hydroxides All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. Oh − is a diatomic anion with the chemical name hydroxide. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The following 95 pages are in this. List Of Hydroxides.
From www.slideserve.com
PPT Chapter Menu PowerPoint Presentation, free download ID5168678 List Of Hydroxides Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. This list may not reflect recent changes. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins.. List Of Hydroxides.
From www.slideserve.com
PPT Solubility of metal hydroxides, and amphoteric behavior List Of Hydroxides All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. Oh − is a diatomic anion with the chemical name hydroxide. This list may not reflect recent changes. The following 95 pages are in this category, out of 95 total. This page discusses the solubility of the hydroxides, sulfates. List Of Hydroxides.
From www.slideserve.com
PPT Lecture 18 (11/29/2006) Systematic Description of Minerals Part 2 List Of Hydroxides Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. The following 95 pages are in this category, out of 95 total. This list may not reflect recent changes. Oh − is a diatomic anion with the chemical name hydroxide. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in. List Of Hydroxides.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry List Of Hydroxides Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. The following 95 pages are in this category, out of 95 total. Oh − is a diatomic anion with the chemical name hydroxide. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This page discusses the solubility. List Of Hydroxides.
From www.chegg.com
Solved Flow Chart 2 The Hydroxides List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. The following 95 pages are in this category, out of 95 total. Oh − is a diatomic anion with the chemical name hydroxide. This page discusses the solubility of the hydroxides, sulfates and carbonates of the. List Of Hydroxides.
From www.slideserve.com
PPT PhD Falfushynska Halina PowerPoint Presentation, free download List Of Hydroxides Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The following 95 pages are in this category, out of. List Of Hydroxides.
From www.sciencephoto.com
Metal hydroxides Stock Image A500/0419 Science Photo Library List Of Hydroxides The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The following 95 pages are in this category, out of 95 total. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This page discusses the solubility of. List Of Hydroxides.
From www.pathwaystochemistry.com
Precipitation Reactions Pathways to Chemistry List Of Hydroxides This list may not reflect recent changes. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. The following 95 pages are in this category, out of 95. List Of Hydroxides.
From studylib.net
Hydroxides M OH List Of Hydroxides The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. The following 95 pages are in this category, out of 95 total. Hydroxide is also. List Of Hydroxides.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium List Of Hydroxides This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases. Oh − is a diatomic anion with the chemical name hydroxide. The following 95 pages are in this category, out of. List Of Hydroxides.
From www.chemistryscl.com
solubility of metal hydroxides in water List Of Hydroxides All hydroxides form at low temperatures and are found predominantly as weathering products, as, for example, from alteration in hydrothermal veins. This list may not reflect recent changes. Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. This page discusses the solubility of the hydroxides, sulfates and carbonates of the group 2 elements—beryllium, magnesium, calcium,. The following 95. List Of Hydroxides.