Threshold Specific Energy . threshold energy = energy of normal molecules + activation energy. (a) threshold energy, (b) energy of activation. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. For a reaction x →y), heat of. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). In this study, an attempt was made to assess the impact. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the.
from www.alamy.com
For a reaction x →y), heat of. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. In this study, an attempt was made to assess the impact. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. threshold energy = energy of normal molecules + activation energy. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). (a) threshold energy, (b) energy of activation.
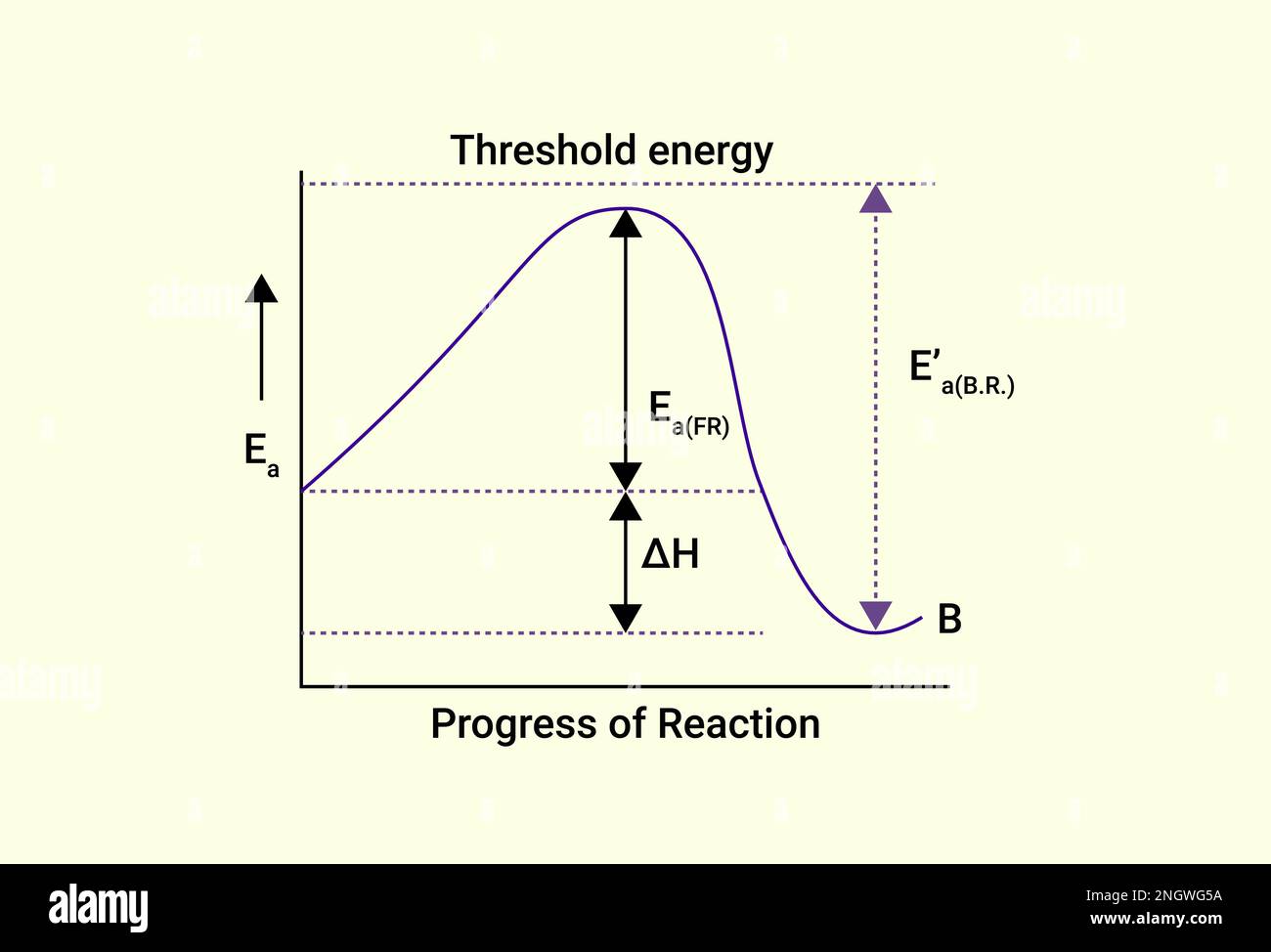
Graph of Progress of Reaction and Threshold energy Stock Vector Image
Threshold Specific Energy while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. For a reaction x →y), heat of. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. (a) threshold energy, (b) energy of activation. In this study, an attempt was made to assess the impact. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. threshold energy = energy of normal molecules + activation energy. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ).
From www.youtube.com
Explain the terms Threshold energy 12 CHEMICAL Threshold Specific Energy the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. while activation energy is specific to a particular reaction, threshold energy is a. Threshold Specific Energy.
From www.researchgate.net
An example of the threshold energy determination for aC sample Threshold Specific Energy threshold energy = energy of normal molecules + activation energy. For a reaction x →y), heat of. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is. Threshold Specific Energy.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Specific Energy (a) threshold energy, (b) energy of activation. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). threshold energy is the minimum. Threshold Specific Energy.
From www.researchgate.net
The threshold displacement energy for ions Download Scientific Diagram Threshold Specific Energy In this study, an attempt was made to assess the impact. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. For a reaction x →y), heat of. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is. Threshold Specific Energy.
From www.researchgate.net
Comparison of specific threshold energy (J/kg). Download Scientific Threshold Specific Energy threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. For a reaction x →y), heat of. threshold energy = energy of normal molecules + activation energy. In this study, an attempt was made to assess the impact. the minimum amount of energy required to remove an electron from. Threshold Specific Energy.
From byjus.com
What is threshold frequency and threshold energy? Threshold Specific Energy the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. For a reaction x →y), heat of. threshold energy is the minimum amount of energy required. Threshold Specific Energy.
From www.mdpi.com
Energies Free FullText EnergyEfficient Advanced Ultrafine Threshold Specific Energy threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. (a) threshold energy, (b) energy of activation. For a reaction x →y), heat of. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. while activation energy. Threshold Specific Energy.
From semiengineering.com
NearThreshold Computing Threshold Specific Energy For a reaction x →y), heat of. (a) threshold energy, (b) energy of activation. In this study, an attempt was made to assess the impact. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. threshold energy is the minimum kinetic energy the molecules must have to. Threshold Specific Energy.
From www.mdpi.com
Materials Free FullText ThresholdVoltage Extraction Methods for Threshold Specific Energy threshold energy = energy of normal molecules + activation energy. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. In this study, an attempt was made to assess the impact. threshold energy is the minimum amount of energy required to initiate a specific process, such. Threshold Specific Energy.
From www.toppr.com
The threshold frequency of a metal is 1 × 10^15sec^1 . The ratio of Threshold Specific Energy threshold energy = energy of normal molecules + activation energy. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. In this study, an. Threshold Specific Energy.
From www.researchgate.net
Threshold energy for breakdown in the spray according to droplet size Threshold Specific Energy the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). (a) threshold energy, (b) energy of activation. In this study, an attempt was made to assess the impact. threshold energy is the minimum amount of energy required to initiate a specific process, such as the. Threshold Specific Energy.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Specific Energy For a reaction x →y), heat of. In this study, an attempt was made to assess the impact. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two.. Threshold Specific Energy.
From www.researchgate.net
Threshold displacement energies (E d ) versus the total amount of Threshold Specific Energy In this study, an attempt was made to assess the impact. threshold energy = energy of normal molecules + activation energy. (a) threshold energy, (b) energy of activation. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. threshold energy is the minimum kinetic energy the. Threshold Specific Energy.
From www.researchgate.net
Temperature dependence of specific voltage Vs and threshold voltage Vt Threshold Specific Energy (a) threshold energy, (b) energy of activation. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. while activation energy is specific to a particular reaction,. Threshold Specific Energy.
From www.researchgate.net
(a) Energy (Er in a.u.) and the threshold energy (E2s in a.u.) vs. 1 Z Threshold Specific Energy threshold energy = energy of normal molecules + activation energy. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. For a reaction x →y), heat of.. Threshold Specific Energy.
From www.researchgate.net
Specific energy thresholds, some for shattering and some for Threshold Specific Energy threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by. Threshold Specific Energy.
From www.nuclear-power.com
Critical Energy Threshold Energy for Fission Threshold Specific Energy threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. For a reaction x →y), heat of. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). threshold energy is the minimum amount of energy required. Threshold Specific Energy.
From www.researchgate.net
Threshold for energy gain based on model calculations. (a) Calculated Threshold Specific Energy while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. In this study, an attempt was made to assess the impact. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). (a) threshold energy, (b) energy. Threshold Specific Energy.
From giogipcdi.blob.core.windows.net
Threshold Impact Energy at Susan Asbury blog Threshold Specific Energy threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy = energy of normal molecules + activation energy. the minimum amount of energy required. Threshold Specific Energy.
From www.researchgate.net
Specific Energy Absorption as a Function of Specific Bending Stiffness Threshold Specific Energy In this study, an attempt was made to assess the impact. threshold energy = energy of normal molecules + activation energy. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). threshold energy is the minimum amount of energy required to initiate a specific. Threshold Specific Energy.
From www.slideserve.com
PPT TeV Neutrinos and Gamma rays from PowerPoint Threshold Specific Energy threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. the minimum amount of energy required to remove an electron from the metal is. Threshold Specific Energy.
From www.researchgate.net
Specific Energy of Each Transfer Orbit Download Scientific Diagram Threshold Specific Energy this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. (a) threshold energy, (b) energy of activation. For a reaction x →y), heat of. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ).. Threshold Specific Energy.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Specific Energy threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. For a reaction x →y), heat of. (a) threshold energy, (b) energy of activation. In this study, an attempt was made to assess the impact. this model allows to analyse the biological effects of radon daughter products on the lung. Threshold Specific Energy.
From www.slideserve.com
PPT NE 301 Introduction to Nuclear Science Spring 2012 PowerPoint Threshold Specific Energy this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to. Threshold Specific Energy.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Specific Energy threshold energy = energy of normal molecules + activation energy. In this study, an attempt was made to assess the impact. For a reaction x →y), heat of. the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). this model allows to analyse the. Threshold Specific Energy.
From www.researchgate.net
Estimation Results of Panel Threshold Model Download Scientific Diagram Threshold Specific Energy threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. the minimum amount of energy required to remove an electron from the metal is. Threshold Specific Energy.
From classnotes.org.in
Arrhenius Equation and Activation Energy Chemical Chemistry Threshold Specific Energy threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. In this study, an attempt was made to assess the impact. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy = energy of normal. Threshold Specific Energy.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Specific Energy For a reaction x →y), heat of. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. while activation energy is specific to a particular reaction, threshold. Threshold Specific Energy.
From www.researchgate.net
(PDF) Threshold Energy Threshold Specific Energy For a reaction x →y), heat of. (a) threshold energy, (b) energy of activation. In this study, an attempt was made to assess the impact. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. while activation energy is specific to a particular reaction, threshold energy is a general concept. Threshold Specific Energy.
From www.researchgate.net
The threshold energy E 0 is determined by the equation (3.1). The red Threshold Specific Energy threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. For a reaction x →y), heat of. In this study, an attempt was made to. Threshold Specific Energy.
From www.researchgate.net
(a) As in figure 4(a) but far from the threshold energy region (ω/I Threshold Specific Energy this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. (a) threshold energy, (b) energy of activation. For a reaction x →y), heat of. In this study, an attempt was made to assess the impact. threshold energy is the minimum kinetic energy the molecules must have to. Threshold Specific Energy.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Specific Energy threshold energy = energy of normal molecules + activation energy. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. (a) threshold energy, (b) energy of activation.. Threshold Specific Energy.
From www.researchgate.net
Probability of detection against energy threshold at different average Threshold Specific Energy while activation energy is specific to a particular reaction, threshold energy is a general concept that applies to any. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and is based on the. threshold energy = energy of normal molecules + activation energy. the minimum amount of energy required. Threshold Specific Energy.
From www.mdpi.com
Materials Free FullText ThresholdVoltage Extraction Methods for Threshold Specific Energy For a reaction x →y), heat of. threshold energy = energy of normal molecules + activation energy. (a) threshold energy, (b) energy of activation. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. In this study, an attempt was made to assess the impact. threshold energy is the. Threshold Specific Energy.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Specific Energy the minimum amount of energy required to remove an electron from the metal is called the threshold energy (denoted by the symbol φ). (a) threshold energy, (b) energy of activation. threshold energy = energy of normal molecules + activation energy. this model allows to analyse the biological effects of radon daughter products on the lung tissue, and. Threshold Specific Energy.