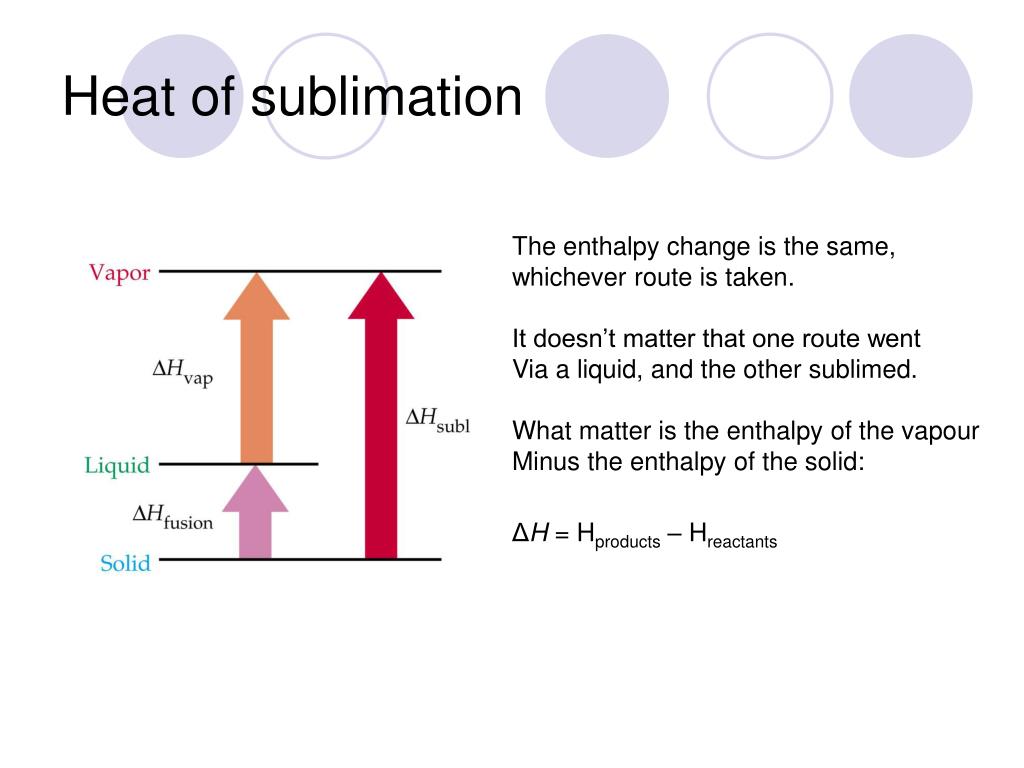
Heat Definition Thermochemistry . Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Amount of energy required to raise the temperature of a given object by 1°c. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The greater the capacity of a body, the more heat it. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy.
from www.slideserve.com
Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The greater the capacity of a body, the more heat it. Amount of energy required to raise the temperature of a given object by 1°c. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,.
PPT Thermochemistry 2 PowerPoint Presentation, free download ID
Heat Definition Thermochemistry Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Amount of energy required to raise the temperature of a given object by 1°c. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. The greater the capacity of a body, the more heat it.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Heat Definition Thermochemistry Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. The greater the capacity of a. Heat Definition Thermochemistry.
From www.scribd.com
Unit 5. Thermochemistry (Answers) 5.2 Define Exothermic and Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Amount of energy required to raise the temperature of a given object by 1°c. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work. Heat Definition Thermochemistry.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Heat Definition Thermochemistry Amount of energy required to raise the temperature of a given object by 1°c. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. The greater the capacity. Heat Definition Thermochemistry.
From studylib.net
Thermochemistry ppt 1/28 Heat Definition Thermochemistry Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Amount of. Heat Definition Thermochemistry.
From studylib.net
Heat Equation Heat Definition Thermochemistry Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Heat (defined later) is random. Heat Definition Thermochemistry.
From slidetodoc.com
Thermochemistry Define Heat Compare Endothermic and Exothermic reactions Heat Definition Thermochemistry Amount of energy required to raise the temperature of a given object by 1°c. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn how thermochemistry describes the energy changes that occur during. Heat Definition Thermochemistry.
From www.slideshare.net
Thermochemistry Heat Definition Thermochemistry Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The greater. Heat Definition Thermochemistry.
From slidetodoc.com
Thermochemistry Define Heat Compare Endothermic and Exothermic reactions Heat Definition Thermochemistry Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. The greater the capacity of a body, the more heat it. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess'. Heat Definition Thermochemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3170116 Heat Definition Thermochemistry Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. The greater the capacity of a body, the more heat it. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn about the role of energy and heat in chemical processes, the principles of. Heat Definition Thermochemistry.
From www.slideshare.net
Thermochemistry Heat Definition Thermochemistry Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Amount of energy required to raise the temperature of a given object by 1°c. The greater the capacity of a body, the more heat it. Learn how to write thermochemical equations, define standard enthalpy of formation, and. Heat Definition Thermochemistry.
From ar.inspiredpencil.com
Heat Definition Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how. Heat Definition Thermochemistry.
From www.youtube.com
Thermochemistry Intro YouTube Heat Definition Thermochemistry Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Amount of energy required to raise the temperature of a given object by 1°c. The greater the capacity of a body, the. Heat Definition Thermochemistry.
From www.slideserve.com
PPT Thermochemistry 2 PowerPoint Presentation, free download ID Heat Definition Thermochemistry Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Amount of energy required to raise the temperature of a given object by 1°c. The greater the capacity. Heat Definition Thermochemistry.
From sciencenotes.org
Exothermic Reactions Definition and Examples Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Amount of energy required to raise the temperature of a given object by 1°c. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical. Heat Definition Thermochemistry.
From slidetodoc.com
Thermochemistry Outline Thermochemical equations Thermodynamic Heat Definition Thermochemistry Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. The greater the capacity of a body, the more heat it. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Amount of energy required to raise the temperature of a. Heat Definition Thermochemistry.
From www.pinterest.com
Explore the Fascinating World of Thermochemistry Heat Definition Thermochemistry Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. The greater the capacity of a body, the more heat it. Amount of energy required to raise the temperature of a given object by 1°c. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical. Heat Definition Thermochemistry.
From www.slideserve.com
PPT THERMOCHEMISTRY Thermodynamics The study of Heat and Work and Heat Definition Thermochemistry Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Amount of energy required to raise the temperature of a given object by 1°c. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. The greater the capacity of a body, the more heat it.. Heat Definition Thermochemistry.
From www.studocu.com
Thermochemistry I. Definition Thermochemistry a. Thermochemistry the Heat Definition Thermochemistry Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn how thermochemistry describes the. Heat Definition Thermochemistry.
From www.difference.minaprem.com
Difference Between Heat and Temperature Heat Definition Thermochemistry Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. The greater the capacity of a body, the more heat it. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Heat (defined later) is random (disorganized) energy that is released. Heat Definition Thermochemistry.
From schoolbag.info
Figure 7.7. Heating Curve for a Single Compound Heat Definition Thermochemistry Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. The greater the capacity of a body, the more heat it. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Amount of energy required to raise the temperature of a. Heat Definition Thermochemistry.
From slidetodoc.com
Thermochemistry Chapter 16 Section 1 Thermochemistry Objectives Define Heat Definition Thermochemistry Amount of energy required to raise the temperature of a given object by 1°c. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Learn how to write thermochemical equations, define. Heat Definition Thermochemistry.
From studylib.net
Thermochemistry Review Answers1 Heat Definition Thermochemistry Amount of energy required to raise the temperature of a given object by 1°c. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Heat (defined later) is random (disorganized) energy that is released or. Heat Definition Thermochemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Thermochemistry is defined as the branch of. Heat Definition Thermochemistry.
From www.youtube.com
Heat of Neutralization Thermochemistry YouTube Heat Definition Thermochemistry Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. The greater the capacity of a body, the more heat it. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Thermochemistry is defined as the branch of. Heat Definition Thermochemistry.
From eduinput.com
ThermochemistryReactions, Systems, Processes, and Aplications Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Amount of energy required to raise the temperature of a given object by 1°c. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work. Heat Definition Thermochemistry.
From www.youtube.com
Thermochemical Equations and Using the energy term (heat of reaction Heat Definition Thermochemistry Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Amount of energy required to raise the temperature of a given object by 1°c. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Heat (defined later) is random (disorganized) energy. Heat Definition Thermochemistry.
From hindi.theindianwire.com
ताप रसायन क्या है? ताप रसायन की जानकारी Thermochemistry in hindi दा Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Learn how to write thermochemical equations, define. Heat Definition Thermochemistry.
From www.slideshare.net
Thermochemistry Heat Definition Thermochemistry Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. Heat (defined later) is random. Heat Definition Thermochemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3170116 Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Amount of energy required to raise the temperature of a given object by 1°c. Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions.. Heat Definition Thermochemistry.
From www.slideshare.net
Thermochemistry Heat Definition Thermochemistry Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. The greater the capacity of a body, the more heat it. Learn about the role of energy and. Heat Definition Thermochemistry.
From treatybottle13.pythonanywhere.com
Divine How To Show Heat In Chemical Equation Reaction Yield Calculator Heat Definition Thermochemistry Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done,. Heat Definition Thermochemistry.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Heat Definition Thermochemistry Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g.,. Heat Definition Thermochemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1281784 Heat Definition Thermochemistry Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Amount of energy required to raise the temperature of a given object by 1°c. Learn how thermochemistry describes the energy changes that occur during chemical reactions and how to measure them using enthalpy,. Learn how to write thermochemical equations, define. Heat Definition Thermochemistry.
From www.slideserve.com
PPT Chapter 16 Reaction Energy PowerPoint Presentation, free download Heat Definition Thermochemistry Thermochemistry is defined as the branch of thermodynamics that focuses on changes occurring during chemical reactions. The greater the capacity of a body, the more heat it. Learn about the role of energy and heat in chemical processes, the principles of thermodynamics, and the concept of enthalpy. Amount of energy required to raise the temperature of a given object by. Heat Definition Thermochemistry.
From www.youtube.com
What's the Difference between Heat and Temperature? YouTube Heat Definition Thermochemistry The greater the capacity of a body, the more heat it. Learn how to write thermochemical equations, define standard enthalpy of formation, and use hess' law and calorimetry to measure. Heat (defined later) is random (disorganized) energy that is released or absorbed, often as a result of some work being done, e.g., sound. Thermochemistry is defined as the branch of. Heat Definition Thermochemistry.