Filtration Distillation Decanting . Decanting is often used to remove hydrated sodium sulfate. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. The video below shows how filtration can be used to separate calcium carbonate. When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Sort them out by particle size using a selective membrane such as filter paper. Particle size can vary considerably, given the type of mixture. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. This process is called decanting, and is the simplest separation method. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different.
from www.numerade.com
When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. This process is called decanting, and is the simplest separation method. The video below shows how filtration can be used to separate calcium carbonate. Particle size can vary considerably, given the type of mixture. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Decanting is often used to remove hydrated sodium sulfate. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. Sort them out by particle size using a selective membrane such as filter paper.
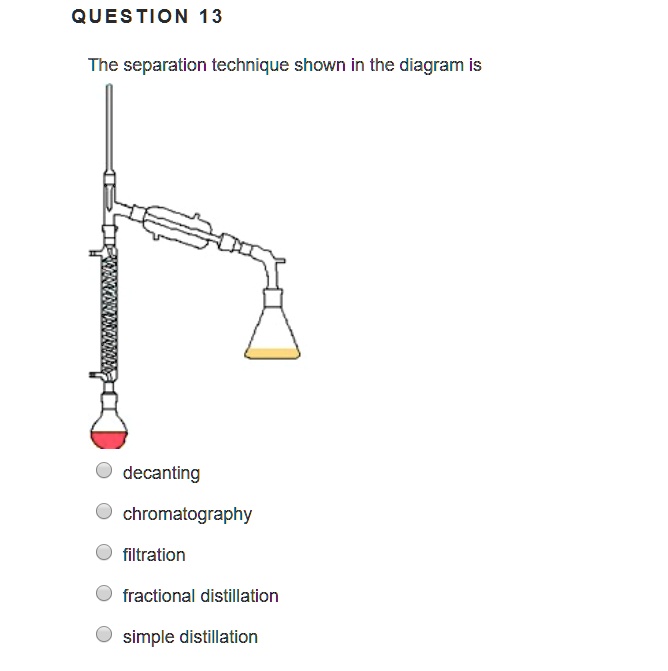
SOLVED QUESTION 13 The separation technique shown in the diagram is
Filtration Distillation Decanting Decanting is often used to remove hydrated sodium sulfate. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. This process is called decanting, and is the simplest separation method. When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Particle size can vary considerably, given the type of mixture. Sort them out by particle size using a selective membrane such as filter paper. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Decanting is often used to remove hydrated sodium sulfate. The video below shows how filtration can be used to separate calcium carbonate. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction.
From www.britannica.com
distillation summary Britannica Filtration Distillation Decanting The video below shows how filtration can be used to separate calcium carbonate. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Sort them out by particle. Filtration Distillation Decanting.
From www.pngegg.com
Filtration Chemistry Mixture Distillation Gas, chemical Element, angle Filtration Distillation Decanting This process is called decanting, and is the simplest separation method. When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the. Filtration Distillation Decanting.
From www.slideserve.com
PPT Centrifugation PowerPoint Presentation, free download ID270173 Filtration Distillation Decanting Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in. Filtration Distillation Decanting.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Filtration Distillation Decanting Particle size can vary considerably, given the type of mixture. The video below shows how filtration can be used to separate calcium carbonate. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. When choosing a technique for separating the components of a. Filtration Distillation Decanting.
From www.researchgate.net
Distillation for Water Purification Download Scientific Diagram Filtration Distillation Decanting Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. The video below shows how filtration can be used to separate calcium carbonate. Decanting is often used to remove hydrated sodium sulfate. When choosing a technique for separating the components. Filtration Distillation Decanting.
From sciencenotes.org
What Is Decantation? Definition and Examples (Chemistry) Filtration Distillation Decanting Decanting is often used to remove hydrated sodium sulfate. Particle size can vary considerably, given the type of mixture. When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are. Filtration Distillation Decanting.
From slidetodoc.com
Methods of Separating Mixtures Filter Decant Evaporation Filtration Distillation Decanting Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. Sort them out by particle size using a selective membrane such as filter paper. Particle size can vary considerably, given the type of mixture. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates. Filtration Distillation Decanting.
From www.slideserve.com
PPT Methods of Separating Mixtures PowerPoint Presentation, free Filtration Distillation Decanting This process is called decanting, and is the simplest separation method. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Decanting is often used to remove hydrated sodium sulfate. Filtration is a separation method used to separate out pure substances in mixtures. Filtration Distillation Decanting.
From ar.inspiredpencil.com
Decantation Process Of Separating Mixtures Filtration Distillation Decanting Decanting is often used to remove hydrated sodium sulfate. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which. Filtration Distillation Decanting.
From www.numerade.com
SOLVED "31. What is the separation technique shown in the diagram C Filtration Distillation Decanting Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase.. Filtration Distillation Decanting.
From www.slideshare.net
Ch 11 8th grade Lessons 1&2 Filtration Distillation Decanting Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. Particle size can vary considerably, given the type of mixture. This process. Filtration Distillation Decanting.
From video.yckmc.edu.hk
Chemistry tutorialCh621Filtration, crystallization, simple Filtration Distillation Decanting Sort them out by particle size using a selective membrane such as filter paper. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation,. Filtration Distillation Decanting.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Filtration Distillation Decanting Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. Filtration is a technique which makes it possible to separate the components of a mixture. Filtration Distillation Decanting.
From slideplayer.com
Mixtures, Elements and Compounds ppt download Filtration Distillation Decanting Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Sort them out by particle size using a selective membrane such as filter paper. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation. Filtration Distillation Decanting.
From www.slideserve.com
PPT Some mixtures can be separated by “pouring off” or Decanting Filtration Distillation Decanting This process is called decanting, and is the simplest separation method. Decanting is often used to remove hydrated sodium sulfate. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be. Filtration Distillation Decanting.
From www.solnpharma.com
Distillation (objective, Principles & Applications) Filtration Distillation Decanting Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Particle size can. Filtration Distillation Decanting.
From dokumen.tips
(PPT) Separations Settling Decanting Filtering Evaporation Filtration Distillation Decanting Decanting is often used to remove hydrated sodium sulfate. The video below shows how filtration can be used to separate calcium carbonate. This process is called decanting, and is the simplest separation method. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different.. Filtration Distillation Decanting.
From www.differencebetween.com
Difference Between Decantation and Filtration Compare the Difference Filtration Distillation Decanting Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. The video below shows how filtration can be used to separate calcium carbonate. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. This process is called decanting,. Filtration Distillation Decanting.
From www.collegesearch.in
What is Decantation Definitions, Examples, Procedure, Applications Filtration Distillation Decanting Sort them out by particle size using a selective membrane such as filter paper. The video below shows how filtration can be used to separate calcium carbonate. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Basic techniques useful for the separation. Filtration Distillation Decanting.
From www.youtube.com
How to separate Mixtures filtration, distillation, sublimation and Filtration Distillation Decanting Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. Filtration is a separation method used to separate out pure substances in. Filtration Distillation Decanting.
From www.careerstoday.in
Separation By Decantation and Loading Careers Today Filtration Distillation Decanting This process is called decanting, and is the simplest separation method. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation. Filtration Distillation Decanting.
From www.youtube.com
SCIENCE GRADE 6 Separating mixtures through Decantation, Evaporation Filtration Distillation Decanting Particle size can vary considerably, given the type of mixture. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. This process is called decanting, and is the simplest separation method. When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. The video. Filtration Distillation Decanting.
From www.goodscience.com.au
Separation of Mixtures Good Science Filtration Distillation Decanting When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Decanting is often used to remove hydrated sodium sulfate. Basic techniques. Filtration Distillation Decanting.
From www.youtube.com
EAU PURE distillation filtration décantation BILAN 6 questions Filtration Distillation Decanting Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. The video below shows how filtration can be used to separate calcium carbonate. This process is called decanting, and is. Filtration Distillation Decanting.
From ar.inspiredpencil.com
Decantation Process Of Separating Mixtures Filtration Distillation Decanting Particle size can vary considerably, given the type of mixture. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. This process is called decanting, and is the simplest separation method. The video below shows how filtration can be used. Filtration Distillation Decanting.
From mavink.com
Filtration Labelled Diagram Filtration Distillation Decanting The video below shows how filtration can be used to separate calcium carbonate. When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be. Filtration Distillation Decanting.
From sciencenotes.org
What Is Decantation? Definition and Examples (Chemistry) Filtration Distillation Decanting Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. Sort them out by particle size using a selective membrane such as filter paper. Fractional distillation is the process of. Filtration Distillation Decanting.
From www.pngegg.com
Chemistry Steam distillation Decantation Solvent in chemical reactions Filtration Distillation Decanting Sort them out by particle size using a selective membrane such as filter paper. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Decanting is often used to remove hydrated sodium sulfate. Basic techniques useful for the separation of mixtures include decantation,. Filtration Distillation Decanting.
From www.slideserve.com
PPT Warmup States of Matter PowerPoint Presentation, free download Filtration Distillation Decanting Sort them out by particle size using a selective membrane such as filter paper. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. The video below shows. Filtration Distillation Decanting.
From ar.inspiredpencil.com
Decantation Diagram Filtration Distillation Decanting This process is called decanting, and is the simplest separation method. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in. Filtration Distillation Decanting.
From www.numerade.com
SOLVED QUESTION 13 The separation technique shown in the diagram is Filtration Distillation Decanting Decanting is often used to remove hydrated sodium sulfate. This process is called decanting, and is the simplest separation method. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation,. Filtration Distillation Decanting.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Filtration Distillation Decanting Particle size can vary considerably, given the type of mixture. This process is called decanting, and is the simplest separation method. Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase. Fractional distillation is the process of. Filtration Distillation Decanting.
From www.youtube.com
Sedimentation,decantation,filtration Process of separation of solid Filtration Distillation Decanting When choosing a technique for separating the components of a mixture, first consider the mixture to be separated, the substance to be. Decanting is often used to remove hydrated sodium sulfate. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Filtration is. Filtration Distillation Decanting.
From mungfali.com
Diagram Of Decantation Filtration Distillation Decanting Sort them out by particle size using a selective membrane such as filter paper. Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. Decanting is often used to remove. Filtration Distillation Decanting.
From slideplayer.com
Methods of Separating Mixtures How can mixtures be separated? ppt Filtration Distillation Decanting Decanting is often used to remove hydrated sodium sulfate. Fractional distillation is the process of separating two or more miscible liquids by a modified distillation process, in which the distillates are collected as fractions having different. Basic techniques useful for the separation of mixtures include decantation, distillation, filtration, evaporation, and extraction. The video below shows how filtration can be used. Filtration Distillation Decanting.