Molecular Models Lab Answer Key . How many pairs of electrons are shared in a double bond? Identify the number of valence electrons of. How does molecule shape change with different numbers of bonds and electron pairs? Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Draw lewis structures for molecules. The goal of this lab is to: Create vsepr models of molecules with molecular. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. You can quickly refer to the periodic table for. Aim to build models of some simple molecules. How many electrons are shared in. Molecular models lab report sheet part i: Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Explore molecule shapes by building molecules in 3d! Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and.
from www.chegg.com
Find out by adding single, double or triple bonds. Draw lewis structures for molecules. You can quickly refer to the periodic table for. Create vsepr models of molecules with molecular. How to draw a lewis dot structure. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Aim to build models of some simple molecules. Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Identify the number of valence electrons of. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization.
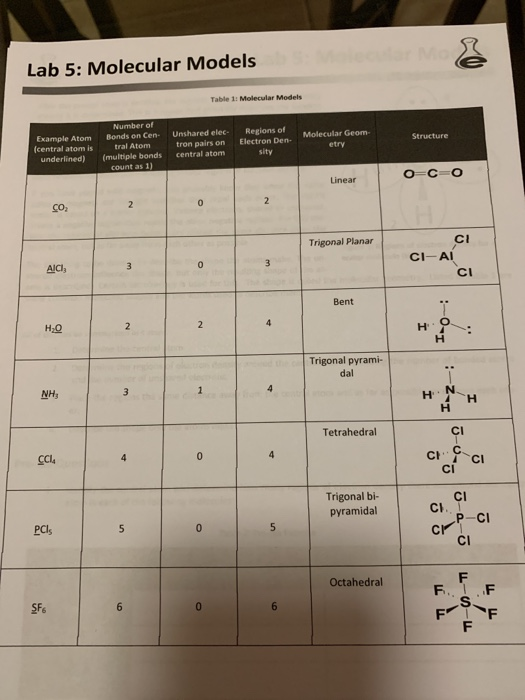
Solved Lab 5 Molecular Models Concepts to explore
Molecular Models Lab Answer Key Molecular models lab report sheet part i: Molecular models lab report sheet part i: Create vsepr models of molecules with molecular. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. How many electrons are shared in. Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Find out by adding single, double or triple bonds. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. How does molecule shape change with different numbers of bonds and electron pairs? Aim to build models of some simple molecules. Draw lewis structures for molecules. The goal of this lab is to: How to draw a lewis dot structure. How many pairs of electrons are shared in a double bond? You can quickly refer to the periodic table for. Explore molecule shapes by building molecules in 3d!
From mariaafsimkins.blob.core.windows.net
Building Molecular Models Lab Answers at mariaafsimkins blog Molecular Models Lab Answer Key Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Identify the number of valence electrons of. Explore molecule shapes by building molecules in 3d! Complete the table and you may use an appropriate set of models to make. Molecular Models Lab Answer Key.
From studyzoneschimmels.z13.web.core.windows.net
Molecular Geometry Worksheets Answers Molecular Models Lab Answer Key Explore molecule shapes by building molecules in 3d! Create vsepr models of molecules with molecular. How to draw a lewis dot structure. Molecular models lab report sheet part i: Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. The goal of this lab is to: Find the total sum of valence electrons that each atom. Molecular Models Lab Answer Key.
From www.studocu.com
Chem 1411 Molecular Models Lab Molecular Models Lab Report Sheet Part Molecular Models Lab Answer Key Create vsepr models of molecules with molecular. How to draw a lewis dot structure. Aim to build models of some simple molecules. Find out by adding single, double or triple bonds. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. How does molecule shape change with different numbers of bonds and electron pairs? You can. Molecular Models Lab Answer Key.
From materialliboster.z19.web.core.windows.net
Molecular Geometry Worksheet Answer Key Molecular Models Lab Answer Key Create vsepr models of molecules with molecular. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Identify the number of valence electrons of. Explore molecule shapes by building molecules in 3d! How many pairs of electrons are shared. Molecular Models Lab Answer Key.
From paytenghopramos.blogspot.com
Molecular Modeling and Lewis Structures Lab Answers Molecular Models Lab Answer Key Aim to build models of some simple molecules. Explore molecule shapes by building molecules in 3d! Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Find out by adding single, double or triple bonds. You can quickly refer to the periodic table for. Identify the number of valence. Molecular Models Lab Answer Key.
From www.studocu.com
Orgo Lab 6 Lab report Lab 6 Molecular Modelling Introduction Molecular Models Lab Answer Key How many electrons are shared in. Explore molecule shapes by building molecules in 3d! Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Identify the number of valence electrons of. Molecular models lab report sheet part i: Draw lewis structures for molecules. Aim to build models of some. Molecular Models Lab Answer Key.
From www.chegg.com
Solved Molecular Shapes 01/22/16 Model5 Name Date Lab Molecular Models Lab Answer Key Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Draw lewis structures for molecules. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Find out by adding single, double or triple bonds. Create vsepr models of molecules with molecular. Formula lewis structure electron. Molecular Models Lab Answer Key.
From www.chegg.com
Solved Using an appropriate set of models, make molecular Molecular Models Lab Answer Key How many electrons are shared in. Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. How many pairs of electrons are shared in a double bond? Now that we have an. Molecular Models Lab Answer Key.
From printablefulldiota.z13.web.core.windows.net
Molecular Geometry Worksheet Pdf Molecular Models Lab Answer Key Molecular models lab report sheet part i: Create vsepr models of molecules with molecular. Find out by adding single, double or triple bonds. How many pairs of electrons are shared in a double bond? Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. You can quickly refer to the periodic table for.. Molecular Models Lab Answer Key.
From www.studocu.com
Online Molecular Models Molecular Models Lab Report Sheet Part I Molecular Models Lab Answer Key How does molecule shape change with different numbers of bonds and electron pairs? You can quickly refer to the periodic table for. Draw lewis structures for molecules. Explore molecule shapes by building molecules in 3d! Identify the number of valence electrons of. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Now. Molecular Models Lab Answer Key.
From studylib.net
Molecular Models Full Lab Purpose To investigate the three Molecular Models Lab Answer Key How does molecule shape change with different numbers of bonds and electron pairs? How to draw a lewis dot structure. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Find out by adding single, double or triple bonds. Draw lewis structures for molecules. How many pairs of electrons are shared in a. Molecular Models Lab Answer Key.
From printablelibwaneer.z19.web.core.windows.net
Lewis Structure Worksheet 1 Answer Key Molecular Models Lab Answer Key Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Explore molecule shapes by building molecules in 3d! Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. The goal of this lab is to: Identify the number of valence electrons. Molecular Models Lab Answer Key.
From www.chegg.com
Solved CHEM 121 Lab Moal Revised 05 2017 Chemical Bonding Molecular Models Lab Answer Key The goal of this lab is to: Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Identify the number of valence electrons of. Molecular models lab report sheet part i: Find out by adding single, double or triple bonds. How does molecule shape change with different numbers of. Molecular Models Lab Answer Key.
From mrklotzswebpage.weebly.com
Bonding, Chemical Nomenclature, and VSEPR GT Mr. Klotz's Page Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. You can quickly refer to the periodic table for. Explore molecule shapes by building molecules in 3d! How many electrons are shared in. Find out by adding single, double or triple bonds. Create vsepr models of molecules with molecular. How does molecule. Molecular Models Lab Answer Key.
From www.housview.com
Molecular Geometry Worksheet Answer Key On Molecular Models Free Molecular Models Lab Answer Key How does molecule shape change with different numbers of bonds and electron pairs? Explore molecule shapes by building molecules in 3d! Molecular models lab report sheet part i: Identify the number of valence electrons of. You can quickly refer to the periodic table for. How many electrons are shared in. How to draw a lewis dot structure. Create vsepr models. Molecular Models Lab Answer Key.
From ar.inspiredpencil.com
Shapes Of Molecules Worksheet Molecular Models Lab Answer Key Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Draw lewis structures for molecules. Create vsepr models of molecules with molecular. How many electrons are shared in. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Identify the number of valence electrons of. Explore molecule shapes. Molecular Models Lab Answer Key.
From www.studypool.com
SOLUTION CHEM 10 SMC Lewis Structures and Molecular Shapes Worksheet Molecular Models Lab Answer Key How to draw a lewis dot structure. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Identify the number of valence electrons of. Create vsepr models of molecules with molecular. Explore molecule shapes by building molecules in. Molecular Models Lab Answer Key.
From www.coursehero.com
[Solved] Lab 10 Molecular Geometry Lab Lewis Structure Molecular Molecular Models Lab Answer Key How many electrons are shared in. Find out by adding single, double or triple bonds. You can quickly refer to the periodic table for. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Aim to build models of some simple molecules. The goal of this lab is to: Formula lewis structure. Molecular Models Lab Answer Key.
From printablevogler.z4.web.core.windows.net
Molecular Geometry Worksheet Answer Key Molecular Models Lab Answer Key Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Draw lewis structures for molecules. How does molecule shape change with different numbers of bonds and electron pairs? Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. How to draw a lewis dot structure.. Molecular Models Lab Answer Key.
From answerfullcopeland.z21.web.core.windows.net
Vsepr Molecular Models Lab Answer Key Molecular Models Lab Answer Key Aim to build models of some simple molecules. Find out by adding single, double or triple bonds. How many pairs of electrons are shared in a double bond? Explore molecule shapes by building molecules in 3d! How to draw a lewis dot structure. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules. Molecular Models Lab Answer Key.
From www.studocu.com
Constructing Molecular Models Lab Studocu Molecular Models Lab Answer Key Molecular models lab report sheet part i: The goal of this lab is to: Identify the number of valence electrons of. How many electrons are shared in. How many pairs of electrons are shared in a double bond? You can quickly refer to the periodic table for. Complete the table and you may use an appropriate set of models to. Molecular Models Lab Answer Key.
From www.chegg.com
Solved Molecular Models Lab Report Sheet Name Quiz is 20 Molecular Models Lab Answer Key Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Explore molecule shapes by building molecules in 3d! How to draw a lewis dot structure. Create vsepr models of molecules with molecular. Draw lewis structures for molecules. Formula lewis structure electron geometry shape of the bond polarity of polar. Molecular Models Lab Answer Key.
From loenfdebo.blob.core.windows.net
Ap Chemistry Molecular Model Lab Answers at Howard Westra blog Molecular Models Lab Answer Key How many pairs of electrons are shared in a double bond? How to draw a lewis dot structure. Explore molecule shapes by building molecules in 3d! How does molecule shape change with different numbers of bonds and electron pairs? Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Find the total sum of valence electrons. Molecular Models Lab Answer Key.
From mariaafsimkins.blob.core.windows.net
Building Molecular Models Lab Answers at mariaafsimkins blog Molecular Models Lab Answer Key Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. The goal of this lab is to: Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. How many pairs of electrons are shared in a double bond? How does molecule shape. Molecular Models Lab Answer Key.
From goodimg.co
️Molecular Models Worksheet Answers Free Download Goodimg.co Molecular Models Lab Answer Key The goal of this lab is to: Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Molecular models lab report sheet part i: You can quickly refer to the periodic table for. Aim to build models of some simple molecules. Create vsepr models of molecules with molecular. Draw lewis structures for. Molecular Models Lab Answer Key.
From studylib.net
Molecular Models Lab Molecular Models Lab Answer Key How many pairs of electrons are shared in a double bond? Molecular models lab report sheet part i: Aim to build models of some simple molecules. Create vsepr models of molecules with molecular. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Identify the number of valence electrons of. How many. Molecular Models Lab Answer Key.
From www.chegg.com
Solved Lab 5 Molecular Models Concepts to explore Molecular Models Lab Answer Key Draw lewis structures for molecules. Create vsepr models of molecules with molecular. Explore molecule shapes by building molecules in 3d! Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. The goal of this lab is to: Identify the number of valence electrons of. Molecular models lab report sheet part i: Now that we have an. Molecular Models Lab Answer Key.
From studylib.net
Molecular Models Lab Molecular Models Lab Answer Key You can quickly refer to the periodic table for. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. How to draw a lewis dot structure. How does molecule shape change with different numbers of bonds and electron pairs? How many electrons are shared in. Find out by adding single, double or. Molecular Models Lab Answer Key.
From answerfullallan101.z19.web.core.windows.net
Molecular Shapes Simulation Worksheet Answers Molecular Models Lab Answer Key Molecular models lab report sheet part i: Explore molecule shapes by building molecules in 3d! How many electrons are shared in. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. How many pairs of electrons are shared in a double bond? Create vsepr models of molecules with molecular. Complete the table and you may use. Molecular Models Lab Answer Key.
From www.studocu.com
Matias psy193 Laboratory 4 molecular geometry Name Section Date Molecular Models Lab Answer Key Molecular models lab report sheet part i: Explore molecule shapes by building molecules in 3d! Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. How does molecule shape change with different numbers of bonds and electron pairs? You can quickly refer to the periodic table for. Create vsepr models of molecules. Molecular Models Lab Answer Key.
From www.chegg.com
Solved Lab 5 Molecular Models Concepts to explore Molecular Models Lab Answer Key Find out by adding single, double or triple bonds. Draw lewis structures for molecules. How many electrons are shared in. Aim to build models of some simple molecules. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. How does molecule shape change with different numbers of bonds and electron pairs? How to draw a lewis. Molecular Models Lab Answer Key.
From www.docsity.com
MOLECULAR GEOMETRY AND Polarity ANSWER KEY Exams Chemistry Docsity Molecular Models Lab Answer Key Molecular models lab report sheet part i: Complete the table and you may use an appropriate set of models to make or observe molecular models of the compounds. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. Create vsepr models of molecules with molecular. How to draw a lewis dot structure.. Molecular Models Lab Answer Key.
From myans.bhantedhammika.net
Shapes Of Molecules Lab Answers Molecular Models Lab Answer Key The goal of this lab is to: You can quickly refer to the periodic table for. Draw lewis structures for molecules. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Molecular models lab report sheet part i: Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Create. Molecular Models Lab Answer Key.
From mariaafsimkins.blob.core.windows.net
Building Molecular Models Lab Answers at mariaafsimkins blog Molecular Models Lab Answer Key How many electrons are shared in. Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and. How many pairs of electrons are shared in a double bond? Molecular models lab report sheet part i: Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. The. Molecular Models Lab Answer Key.
From goodimg.co
️Molecular Models Worksheet Answers Free Download Goodimg.co Molecular Models Lab Answer Key Aim to build models of some simple molecules. Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Formula lewis structure electron geometry shape of the bond polarity of polar or hybridization. Explore molecule shapes by building molecules in 3d! Now that we have an understanding of covalent bonding and how atoms share. Molecular Models Lab Answer Key.