Dilution Practice Problems With Answers . Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. What is the nitrate ion. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. See examples of calculating molarity, volume and amount of. See examples of dilution problems with. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. Learn how to set up and solve the. 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. What was the beginning volume of the 20% solution? 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. Dilution m 1v 1=m 2v 2 1. Learn how to solve dilution problems using the equation m1v1 = m2v2.
from www.chegg.com
Learn how to set up and solve the. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. Learn how to solve dilution problems using the equation m1v1 = m2v2. What is the nitrate ion. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. Dilution m 1v 1=m 2v 2 1. See examples of dilution problems with. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. See examples of calculating molarity, volume and amount of.
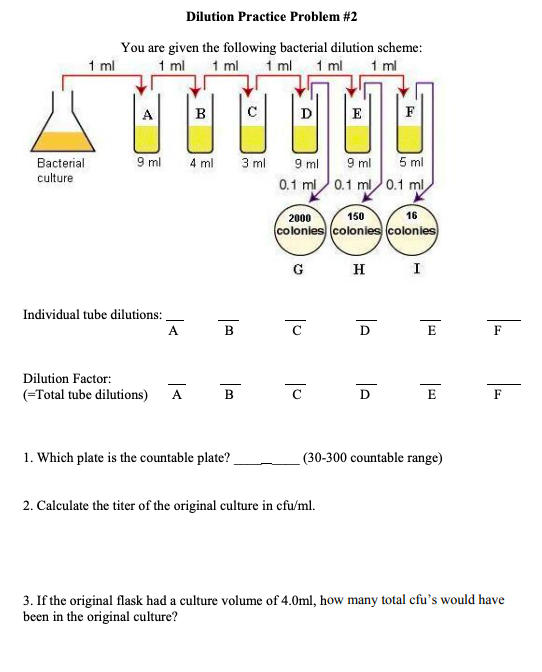
Solved Dilution Practice Problem 2 You are given the
Dilution Practice Problems With Answers Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. There’s a bottle of 0.750 m nacl on a shelf. 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. Learn how to solve dilution problems using the equation m1v1 = m2v2. Dilution m 1v 1=m 2v 2 1. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. How much of it do you need to prepare 50 ml of a. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. See examples of calculating molarity, volume and amount of. What was the beginning volume of the 20% solution? See examples of dilution problems with. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution. Learn how to set up and solve the. What is the nitrate ion. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution.
From answerlistsybil.z13.web.core.windows.net
Dilution Practice Problems Worksheet Answers Dilution Practice Problems With Answers Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to set up and solve the. What was the beginning volume of the 20% solution? 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. Dilution m 1v 1=m 2v 2 1. Test your. Dilution Practice Problems With Answers.
From worksheets.clipart-library.com
Molarity and Dilutions Practice Worksheet Key Exercises Dilution Practice Problems With Answers 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. Learn how to set up and solve the. See examples of dilution problems with. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to calculate the molarity of a dilute solution by adding. Dilution Practice Problems With Answers.
From www.chegg.com
Solved More Dilution Practice Problems!!10kg of baking soda Dilution Practice Problems With Answers What was the beginning volume of the 20% solution? Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. How much of it do you need to prepare 50 ml of a. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. There’s a bottle of 0.750 m nacl on a. Dilution Practice Problems With Answers.
From answerlistsybil.z13.web.core.windows.net
Dilution Practice Problems Worksheet Answers Dilution Practice Problems With Answers Learn how to solve dilution problems using the equation m1v1 = m2v2. There’s a bottle of 0.750 m nacl on a shelf. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. See examples of dilution problems with. Learn how to set up and solve the. 46.2 ml of a 0.568 m ca(no 3). Dilution Practice Problems With Answers.
From www.youtube.com
Solving dilution problems YouTube Dilution Practice Problems With Answers Learn how to solve dilution problems using the equation m1v1 = m2v2. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to set up and solve the. 10% a 20% solution has been diluted to 400 ml and. Dilution Practice Problems With Answers.
From www.chegg.com
Solved PROBLEMS Practice calculating serial dilutions using Dilution Practice Problems With Answers What is the nitrate ion. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution. Learn how to set up and solve the. Test your understanding of molarity, molar mass, and dilution with these practice problems. Dilution Practice Problems With Answers.
From www.studocu.com
Conversions and Dilutions Practice Problems Answer Key Practice Dilution Practice Problems With Answers Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. How much of it do you need to prepare 50 ml of a. What was the beginning volume of the 20% solution? See examples of dilution problems with. Learn how to. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution Practice Problem MI You are given the Dilution Practice Problems With Answers Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. What is the nitrate ion. See examples of calculating molarity, volume and amount of. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride. Dilution Practice Problems With Answers.
From worksheetkrause.z19.web.core.windows.net
Dilution Problems Chemistry Worksheet Dilution Practice Problems With Answers Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. See examples of calculating molarity, volume and amount of. 15 practice problem a stock. Dilution Practice Problems With Answers.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Dilution Practice Problems With Answers What is the nitrate ion. Learn how to set up and solve the. What was the beginning volume of the 20% solution? Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution.. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Practice Dilution Problems Section II 1. Beginning Dilution Practice Problems With Answers Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of it do you need to prepare 50 ml of a. What is the nitrate ion. Dilution m 1v 1=m 2v 2 1. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. 10% a 20% solution has been diluted. Dilution Practice Problems With Answers.
From davida.davivienda.com
Dilution Practice Problems Worksheet Answers Printable Word Searches Dilution Practice Problems With Answers 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to set up and solve the. Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50 ml of. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Practice Plate Count Dilution Problem \1 A sample of Dilution Practice Problems With Answers 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. What was the beginning volume of the 20% solution? Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. What is the nitrate ion. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. Dilution. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution Dilution Practice Problems With Answers What is the nitrate ion. See examples of dilution problems with. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. See examples of calculating molarity, volume and amount of. 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution. There’s a bottle of 0.750 m. Dilution Practice Problems With Answers.
From www.studocu.com
Dilution problems and answers (for practice) CHEM 1030 Studocu Dilution Practice Problems With Answers 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. There’s a bottle of 0.750 m nacl on a shelf. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of it. Dilution Practice Problems With Answers.
From www.chegg.com
Solved 4. Practice dilution problems a. Amy counted 40 Dilution Practice Problems With Answers How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution.. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution Practice ProblemsSolve the following Dilution Practice Problems With Answers Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. Dilution m 1v 1=m 2v 2 1. See examples of calculating molarity, volume and amount of. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. Learn how to set up and solve the. 15 practice problem a. Dilution Practice Problems With Answers.
From learninglibrarylinton.z21.web.core.windows.net
Dilution Practice Problems Worksheet Answers Dilution Practice Problems With Answers Dilution m 1v 1=m 2v 2 1. What is the nitrate ion. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. See examples of calculating molarity, volume and amount of. Learn how to solve dilution problems using the equation. Dilution Practice Problems With Answers.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Practice Problems With Answers Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. There’s a bottle of 0.750 m nacl on a shelf. See examples of dilution problems with. How much of it do you need to prepare 50 ml of a. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. 10% a. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution Practice Problems 1. If a broth contained Dilution Practice Problems With Answers What was the beginning volume of the 20% solution? There’s a bottle of 0.750 m nacl on a shelf. Learn how to solve dilution problems using the equation m1v1 = m2v2. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to set up and solve the. See examples of dilution problems with. 10% a. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution Practice Problem 3 You are given the Dilution Practice Problems With Answers Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. 10% a 20% solution has been diluted to 400 ml and is now a. Dilution Practice Problems With Answers.
From classfullkay.z13.web.core.windows.net
Molarity By Dilution Worksheets Answers Dilution Practice Problems With Answers Dilution m 1v 1=m 2v 2 1. See examples of calculating molarity, volume and amount of. What was the beginning volume of the 20% solution? Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. There’s a bottle of 0.750 m nacl on a shelf. 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed. Dilution Practice Problems With Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Practice Problems With Answers Learn how to set up and solve the. How much of it do you need to prepare 50 ml of a. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. There’s a bottle of 0.750 m nacl on a shelf. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution Practice Problem 5 You are given the Dilution Practice Problems With Answers Dilution m 1v 1=m 2v 2 1. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. How much of it do you need to prepare 50 ml of a. What is the. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Dilution Practice Problems With Answers What is the nitrate ion. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. See examples of calculating molarity, volume and amount of. See examples of dilution problems with. Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of it do you need to prepare 50 ml of a. What was. Dilution Practice Problems With Answers.
From www.chegg.com
Solved PRACTICE SERIAL DILUTION PROBLEMS Assume the Dilution Practice Problems With Answers 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution. See examples of calculating molarity, volume and amount of. How much of it do you need to prepare 50 ml of a. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. Test. Dilution Practice Problems With Answers.
From www.chegg.com
SERIAL DILUTIONS PRACTICE PROBLEMO If the final Dilution Practice Problems With Answers 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. Learn how to solve dilution problems using the equation m1v1 = m2v2. Practice dilutions problems with answers using nabr, lithium acetate and sodium chloride solutions. Learn how to set up and solve the. How much of it do you need to prepare 50 ml. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution practice problems 1. 'Calculate CFU/ml of Dilution Practice Problems With Answers 15 practice problem a stock solution of hydrochloric acid is 23% hcl by weight and has a density of 1.2 g/ml. Dilution m 1v 1=m 2v 2 1. What is the nitrate ion. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. How much of it do you need to prepare 50 ml. Dilution Practice Problems With Answers.
From lessondbjack.z13.web.core.windows.net
Dilutions Worksheet Chemistry Answers Dilution Practice Problems With Answers 46.2 ml of a 0.568 m ca(no 3) 2 solution is mixed with 80.5 ml of 1.396 m calcium nitrate solution. What was the beginning volume of the 20% solution? See examples of dilution problems with. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. See examples of calculating molarity, volume and amount. Dilution Practice Problems With Answers.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Dilution Practice Problems With Answers See examples of calculating molarity, volume and amount of. What is the nitrate ion. See examples of dilution problems with. How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. 15 practice problem. Dilution Practice Problems With Answers.
From thekidsworksheet.com
Dilutions Worksheet Answer Key Thekidsworksheet Dilution Practice Problems With Answers Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. How much of it do you need to prepare 50 ml of a. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. What is the nitrate ion. 15 practice problem a stock solution of hydrochloric acid is. Dilution Practice Problems With Answers.
From studydbharold77.s3-website-us-east-1.amazonaws.com
Dilution Practice Problems Worksheets Answers Dilution Practice Problems With Answers Learn how to solve dilution problems using the equation m1v1 = m2v2. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. There’s a bottle of 0.750 m nacl on a shelf. What was the beginning volume of the 20% solution? 15 practice problem a stock solution of hydrochloric acid is 23% hcl by. Dilution Practice Problems With Answers.
From www.chegg.com
Solved This problem set should help you practice dilution Dilution Practice Problems With Answers Learn how to set up and solve the. Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. Learn how to solve dilution problems using the equation m1v1 = m2v2. What was the beginning volume of the 20%. Dilution Practice Problems With Answers.
From www.chegg.com
Solved Dilution Practice Problem 2 You are given the Dilution Practice Problems With Answers Test your understanding of molarity, molar mass, and dilution with these practice problems on solution concentration. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to set up and solve the. Learn how to calculate the molarity of a dilute solution by adding more solvent without more solute. How much of it do you need. Dilution Practice Problems With Answers.
From davida.davivienda.com
Dilution Practice Problems Worksheet Answers Printable Word Searches Dilution Practice Problems With Answers How much of it do you need to prepare 50 ml of a. See examples of calculating molarity, volume and amount of. Learn how to solve dilution problems using the equation m1v1 = m2v2. 10% a 20% solution has been diluted to 400 ml and is now a 5% solution. Practice dilutions problems with answers using nabr, lithium acetate and. Dilution Practice Problems With Answers.