Eu Mdr Medical Device Definition . proposal for a regulation of the european parliament and of the council amending regulations (eu). the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must.
from clin-r.com
the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. proposal for a regulation of the european parliament and of the council amending regulations (eu). In the european union (eu) they must.
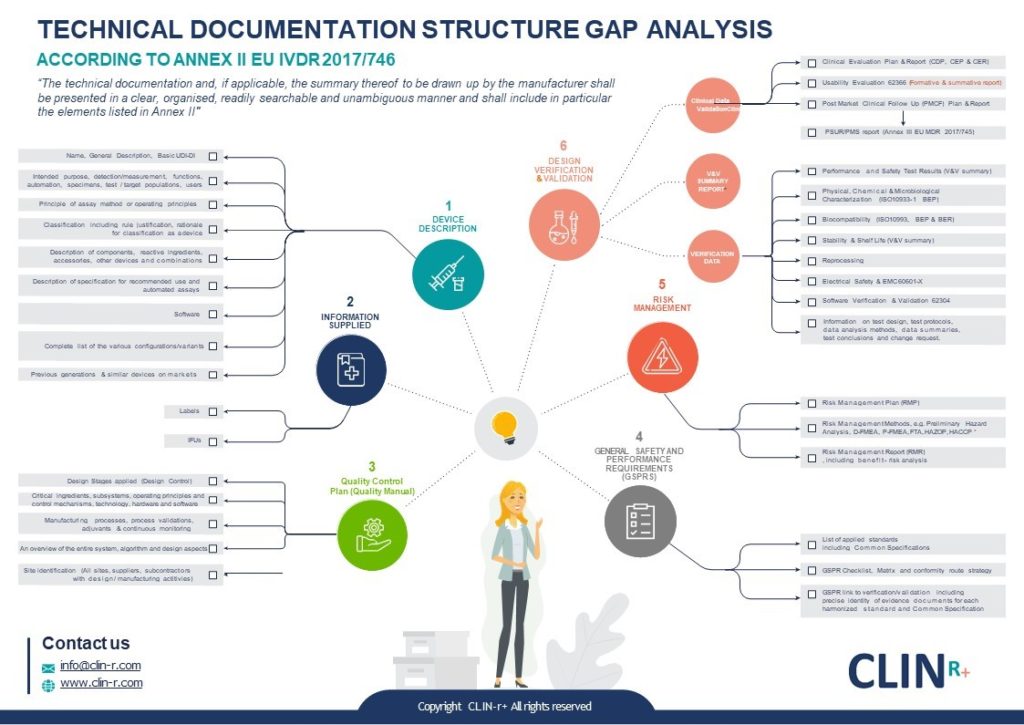
EU MDR how to structure your Medical Device Technical Document Clin R
Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. proposal for a regulation of the european parliament and of the council amending regulations (eu). (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal.
From operonstrategist.com
Medical Device Classification EU MDR Guide) Operon Strategist Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. medical devices are products or equipment intended. Eu Mdr Medical Device Definition.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical. Eu Mdr Medical Device Definition.
From clin-r.com
EU MDR how to structure your Medical Device Technical Document Clin R Eu Mdr Medical Device Definition regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. In the european. Eu Mdr Medical Device Definition.
From naala.nl
What is the difference between the EU MDR and EU IVDR? NAALA Eu Mdr Medical Device Definition regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. In the european. Eu Mdr Medical Device Definition.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Eu Mdr Medical Device Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. medical devices are products or equipment intended. Eu Mdr Medical Device Definition.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Eu Mdr Medical Device Definition medical devices are products or equipment intended for a medical purpose. proposal for a regulation of the european parliament and of the council amending regulations (eu). the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent,. Eu Mdr Medical Device Definition.
From www.greenlight.guru
How are Medical Devices Classified under EU MDR? Eu Mdr Medical Device Definition medical devices are products or equipment intended for a medical purpose. proposal for a regulation of the european parliament and of the council amending regulations (eu). In the european union (eu) they must. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means. Eu Mdr Medical Device Definition.
From www.presentationeze.com
MDR Medical Device Regulation EU 2017 745 Timeline PresentationEZE Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. medical devices are products or equipment intended for a medical purpose. proposal for a regulation of the european parliament and of the council amending regulations (eu). In the european union (eu) they must. (1) ‘medical device’ means any instrument, apparatus, appliance,. Eu Mdr Medical Device Definition.
From emmainternational.com
Classifying Medical Devices under EU MDR Eu Mdr Medical Device Definition the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. proposal for a regulation of the european parliament and of the council amending regulations (eu). In the european. Eu Mdr Medical Device Definition.
From www.youtube.com
CE Marking Process as per EU MDR (European Medical Device Regulation Eu Mdr Medical Device Definition the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. medical devices are products or equipment intended. Eu Mdr Medical Device Definition.
From simplerqms.com
EU MDR Medical Device Classification Classes and Examples Eu Mdr Medical Device Definition proposal for a regulation of the european parliament and of the council amending regulations (eu). the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. medical devices are products or equipment intended. Eu Mdr Medical Device Definition.
From www.slideshare.net
Medical Devices Regulation (MDR) 2017/745 Part I Purpose, Scope Eu Mdr Medical Device Definition proposal for a regulation of the european parliament and of the council amending regulations (eu). medical devices are products or equipment intended for a medical purpose. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent,. Eu Mdr Medical Device Definition.
From www.universalmedica.com
Key Aspects of New EU Medical Devices Regulation (EU 2017/745 Eu Mdr Medical Device Definition medical devices are products or equipment intended for a medical purpose. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the complete definition of the term medical device is laid down in article 2 (1). Eu Mdr Medical Device Definition.
From advisera.com
EU MDR vs. MDD Key differences [Infographic] Eu Mdr Medical Device Definition the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. proposal for a regulation of the european. Eu Mdr Medical Device Definition.
From www.shutterstock.com
Mdr Medical Device Regulation Regulation Eu Stock Vector (Royalty Free Eu Mdr Medical Device Definition In the european union (eu) they must. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. proposal for a regulation of the european parliament and of the council amending regulations (eu). regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.. Eu Mdr Medical Device Definition.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU Eu Mdr Medical Device Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. In the european union (eu) they must. proposal for a regulation of the european parliament and of the council amending regulations (eu). the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. . Eu Mdr Medical Device Definition.
From www.regdesk.co
EU MDR overview An Update to European Medical Device Regulations RegDesk Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article.. Eu Mdr Medical Device Definition.
From maxcert.de
EU MDR Process Medical devices Eu Mdr Medical Device Definition In the european union (eu) they must. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. proposal for a regulation of the european parliament and of the council amending regulations (eu). . Eu Mdr Medical Device Definition.
From www.cosmotrace.com
Medical Devices EU regulations for MDR Part 1 Cosmotrace Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. proposal for a regulation of the european parliament and of the council amending regulations (eu). the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus,. Eu Mdr Medical Device Definition.
From www.mantrasystems.co.uk
Software as a Medical Device (SaMD) for the EU MDR Eu Mdr Medical Device Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. In the european union (eu) they must.. Eu Mdr Medical Device Definition.
From simplerqms.com
EU MDR Medical Device Classification Classes and Examples Eu Mdr Medical Device Definition medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. Eu Mdr Medical Device Definition.
From www.jamasoftware.com
Takeaways What Changes to the EU MDR Mean for You Jama Software Eu Mdr Medical Device Definition proposal for a regulation of the european parliament and of the council amending regulations (eu). In the european union (eu) they must. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of. Eu Mdr Medical Device Definition.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Eu Mdr Medical Device Definition In the european union (eu) they must. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and. Eu Mdr Medical Device Definition.
From www.iascertification.com
EUMDR Certification Medical Device Regulation IAS Eu Mdr Medical Device Definition In the european union (eu) they must. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. . Eu Mdr Medical Device Definition.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Eu Mdr Medical Device Definition In the european union (eu) they must. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices are products or equipment intended for a medical purpose. proposal for a. Eu Mdr Medical Device Definition.
From paragon-cert.com
EU MDR certification Eu Mdr Medical Device Definition In the european union (eu) they must. proposal for a regulation of the european parliament and of the council amending regulations (eu). the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. . Eu Mdr Medical Device Definition.
From medrio.com
European MDR (EU MDR) Guide to Prepare Eu Mdr Medical Device Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. medical devices are products or equipment intended for a medical purpose. the complete definition of the term medical device is laid down in article 2 (1). Eu Mdr Medical Device Definition.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. In the european union (eu) they must. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article.. Eu Mdr Medical Device Definition.
From www.extrahorizon.com
What does the EU MDR mean for your medical device product? Eu Mdr Medical Device Definition medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. proposal for a regulation of the european parliament and of the council amending regulations (eu). the complete definition of the term medical device is laid. Eu Mdr Medical Device Definition.
From www.kolabtree.com
Prepare your medical device for EU MDR 8 trusted resources Eu Mdr Medical Device Definition medical devices are products or equipment intended for a medical purpose. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. proposal for a regulation of the european parliament and of the council amending regulations (eu). In the european union (eu) they must. (1) ‘medical device’ means. Eu Mdr Medical Device Definition.
From www.regulatoryglobe.com
EU MDR implementation guide for medical devices MDCG Eu Mdr Medical Device Definition proposal for a regulation of the european parliament and of the council amending regulations (eu). regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. medical devices are products or equipment. Eu Mdr Medical Device Definition.
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Eu Mdr Medical Device Definition the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. proposal for a regulation of the. Eu Mdr Medical Device Definition.
From www.jamasoftware.com
What the New Medical Device Regulations (EU MDR) Mean for You Jama Eu Mdr Medical Device Definition medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. the complete definition of the term medical device is laid down in article 2 (1). Eu Mdr Medical Device Definition.
From www.acquiscompliance.com
EU MDR Compliance Key Requirements for Medical Devices Eu Mdr Medical Device Definition the medical device regulation (mdr), which was adopted in april 2017, changes the european legal. In the european union (eu) they must. the complete definition of the term medical device is laid down in article 2 (1) of regulation (eu) 2017/745. medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of. Eu Mdr Medical Device Definition.
From galtmedical.com
What the EU MDR Extension Means for Medical Device Buyers Galt Medical Eu Mdr Medical Device Definition proposal for a regulation of the european parliament and of the council amending regulations (eu). regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal.. Eu Mdr Medical Device Definition.