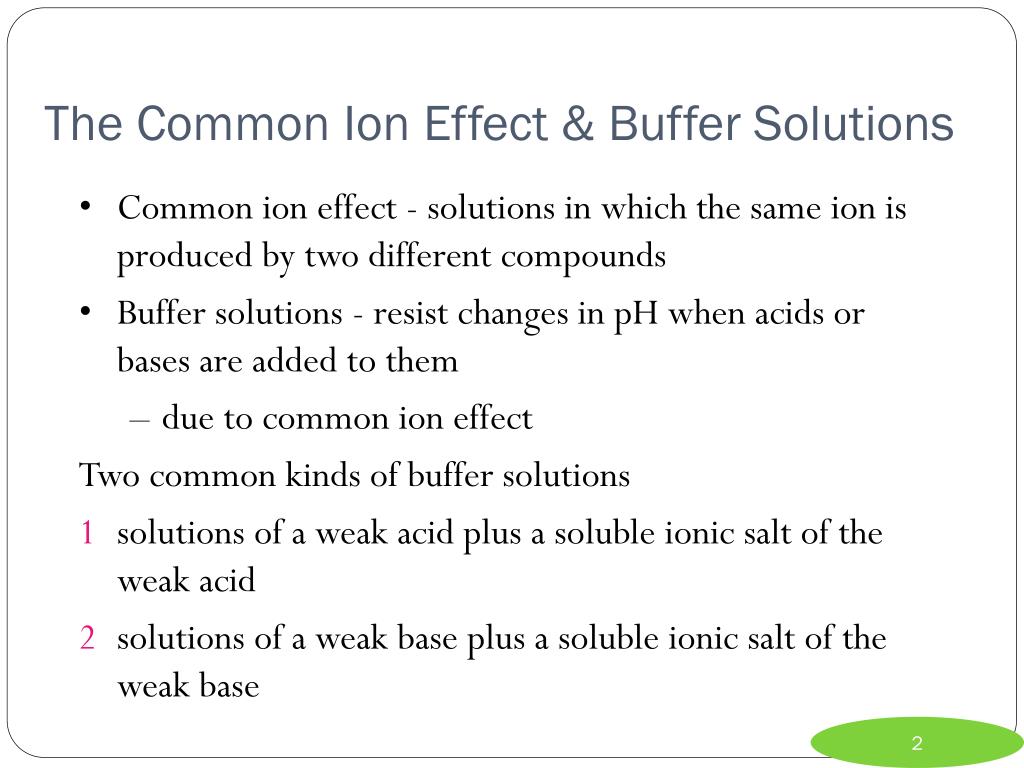
Buffer Effect In Biology . by logan romford. The ph of a buffer. the mechanism involves a buffer, a solution that resists dramatic changes in ph. the aim of this work is to overview the specific effect of ph buffers in biological systems. Buffers are important in biological systems because of their. A buffer (or buffered) solution is one that resists a. you should be aware that buffers play a critical role in almost all biochemical systems. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological buffers are essential for maintaining a stable internal environment in living. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added.
from www.slideserve.com
The ph of a buffer. Buffers are important in biological systems because of their. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. the aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are essential for maintaining a stable internal environment in living. by logan romford. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system.
PPT Buffers and Titrations PowerPoint Presentation, free download ID2923980
Buffer Effect In Biology by logan romford. the mechanism involves a buffer, a solution that resists dramatic changes in ph. by logan romford. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. The ph of a buffer. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer (or buffered) solution is one that resists a. the aim of this work is to overview the specific effect of ph buffers in biological systems. Buffers are important in biological systems because of their. you should be aware that buffers play a critical role in almost all biochemical systems. Biological buffers are essential for maintaining a stable internal environment in living. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Buffer Effect In Biology A buffer (or buffered) solution is one that resists a. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological buffers are essential for maintaining a stable internal environment in living. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are. Buffer Effect In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. the aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are essential for maintaining a stable internal environment in living. you should be aware that buffers play a critical role in almost all biochemical systems.. Buffer Effect In Biology.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Buffer Effect In Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. by logan romford.. Buffer Effect In Biology.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Experiment GoldBio Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. Biological buffers are essential for maintaining a stable internal environment in living. A buffer (or buffered) solution is one that resists a. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma. Buffer Effect In Biology.
From docslib.org
Biological Buffers Reference Chart DocsLib Buffer Effect In Biology the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. by logan romford. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. you should be aware that buffers play a critical role in almost all biochemical systems. Buffers are important. Buffer Effect In Biology.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffer Effect In Biology the aim of this work is to overview the specific effect of ph buffers in biological systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph of a buffer. Buffers are important in biological systems because of their. the most relevant systems for biology are the carbonic. Buffer Effect In Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID3105280 Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph of a buffer. Buffers are important in biological systems because of their. buffers are solutions that moderate ph changes when an acid or base is. Buffer Effect In Biology.
From exoxulnnf.blob.core.windows.net
Biological Roles Of Buffers at Dana Wild blog Buffer Effect In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. the mechanism involves a buffer, a solution that resists dramatic changes in ph. by logan romford. the most relevant systems. Buffer Effect In Biology.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. A buffer (or buffered) solution is one that. Buffer Effect In Biology.
From www.slideshare.net
Buffers in biological systems Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play. Buffer Effect In Biology.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Presentation ID2813988 Buffer Effect In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. Biological buffers are essential for maintaining a stable internal environment in. Buffer Effect In Biology.
From users.highland.edu
Buffers Buffer Effect In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph of a buffer. you should be aware that buffers play a critical role in almost all biochemical systems. Buffers are important in biological systems because of their. by logan romford. the mechanism involves a buffer, a solution. Buffer Effect In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Buffer Effect In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer (or buffered) solution is one that resists a. Buffers are important in biological systems because of their. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers, buffer capacity, and range. Buffer Effect In Biology.
From www.researchgate.net
Specific buffer effects on zeta potential of (A) LYZ (B) SBA15 (C)... Download Scientific Diagram Buffer Effect In Biology Buffers are important in biological systems because of their. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological buffers are essential for maintaining a stable internal environment in living. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added.. Buffer Effect In Biology.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Experiment GoldBio Buffer Effect In Biology Buffers are important in biological systems because of their. Biological buffers are essential for maintaining a stable internal environment in living. A buffer (or buffered) solution is one that resists a. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers are solutions that moderate ph changes when an acid. Buffer Effect In Biology.
From www.tffn.net
What is a Buffer in Science? Exploring the Role of Buffers in Chemistry and Biology The Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the aim of this work is to overview the specific effect of ph buffers in biological systems. buffers, buffer capacity, and range t resist changes in ph. Buffer Effect In Biology.
From www.slideserve.com
PPT CHAPTER 2 Water and Aqueous Solutions PowerPoint Presentation, free download ID1343110 Buffer Effect In Biology A buffer (or buffered) solution is one that resists a. The ph of a buffer. the aim of this work is to overview the specific effect of ph buffers in biological systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers are solutions that moderate ph changes when. Buffer Effect In Biology.
From www.youtube.com
WCLN Buffers Biology YouTube Buffer Effect In Biology you should be aware that buffers play a critical role in almost all biochemical systems. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their. the. Buffer Effect In Biology.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in Buffer Effect In Biology by logan romford. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. Buffers are important in biological systems because of their. Biological buffers are essential for maintaining a stable internal environment in living. A. Buffer Effect In Biology.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffer Effect In Biology The ph of a buffer. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. by logan romford. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their. A buffer (or buffered) solution. Buffer Effect In Biology.
From www.embopress.org
Negative feedback buffers effects of regulatory variants Molecular Systems Biology Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. Biological buffers are essential for maintaining a stable internal environment in living. the aim of this work is to overview the specific effect of ph buffers in biological systems. buffers are solutions that moderate ph changes when an acid or base is added to. Buffer Effect In Biology.
From dxobwrvez.blob.core.windows.net
Common Buffers In Biochemistry at Mario Ross blog Buffer Effect In Biology The ph of a buffer. Buffers are important in biological systems because of their. by logan romford. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. you should be aware that buffers play a critical role in almost all biochemical systems. the aim of this work is. Buffer Effect In Biology.
From www.slideserve.com
PPT Biology Chapter 6 PowerPoint Presentation, free download ID2514063 Buffer Effect In Biology Biological buffers are essential for maintaining a stable internal environment in living. by logan romford. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. you should be aware that buffers play a critical role in almost all biochemical systems. the mechanism involves a buffer, a solution that. Buffer Effect In Biology.
From exohjgjqq.blob.core.windows.net
Buffer Biology Sentence at Valencia Douglas blog Buffer Effect In Biology you should be aware that buffers play a critical role in almost all biochemical systems. The ph of a buffer. Biological buffers are essential for maintaining a stable internal environment in living. Buffers are important in biological systems because of their. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered). Buffer Effect In Biology.
From www.youtube.com
Buffer action in the blood YouTube Buffer Effect In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. by logan romford. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. The ph of a. Buffer Effect In Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID3105280 Buffer Effect In Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer (or buffered) solution is one that resists a. you should be aware that buffers play a critical role in almost all biochemical systems. the aim of this work is to overview the specific effect of ph buffers. Buffer Effect In Biology.
From themedicalbiochemistrypage.net
Ionic Equilibrium Biological Buffering The Medical Biochemistry Page Buffer Effect In Biology the aim of this work is to overview the specific effect of ph buffers in biological systems. Biological buffers are essential for maintaining a stable internal environment in living. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their. buffers,. Buffer Effect In Biology.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Buffer Effect In Biology by logan romford. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. The ph of a buffer. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists a. buffers, buffer capacity, and range t resist changes in. Buffer Effect In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Buffer Effect In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. A buffer (or buffered) solution is one that resists a. you should be aware that buffers play a critical role in almost all biochemical. Buffer Effect In Biology.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download ID2923980 Buffer Effect In Biology buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists a. Buffers are important in biological systems because of their. the aim of this work is. Buffer Effect In Biology.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffer Effect In Biology the mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers are important in biological systems because of their. the aim of this work is to overview the specific effect of ph buffers in biological systems. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system.. Buffer Effect In Biology.
From slideplayer.com
Biology Concepts & Applications ppt download Buffer Effect In Biology buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Biological buffers are essential for maintaining a stable internal environment in living. The ph of a buffer. buffers are solutions that moderate. Buffer Effect In Biology.
From dxopbwbtw.blob.core.windows.net
How Do Buffers Work In Blood at Carter Nolan blog Buffer Effect In Biology the aim of this work is to overview the specific effect of ph buffers in biological systems. A buffer (or buffered) solution is one that resists a. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers are solutions that moderate ph changes when an acid or base is added. Buffer Effect In Biology.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer solutions . PowerPoint Buffer Effect In Biology by logan romford. the aim of this work is to overview the specific effect of ph buffers in biological systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers, buffer capacity, and range t resist changes in ph when small amounts of acid or base are added.. Buffer Effect In Biology.
From www.youtube.com
Buffers and pH Biology YouTube Buffer Effect In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the aim of this work is to overview the specific effect of ph buffers in biological systems. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer (or buffered) solution. Buffer Effect In Biology.