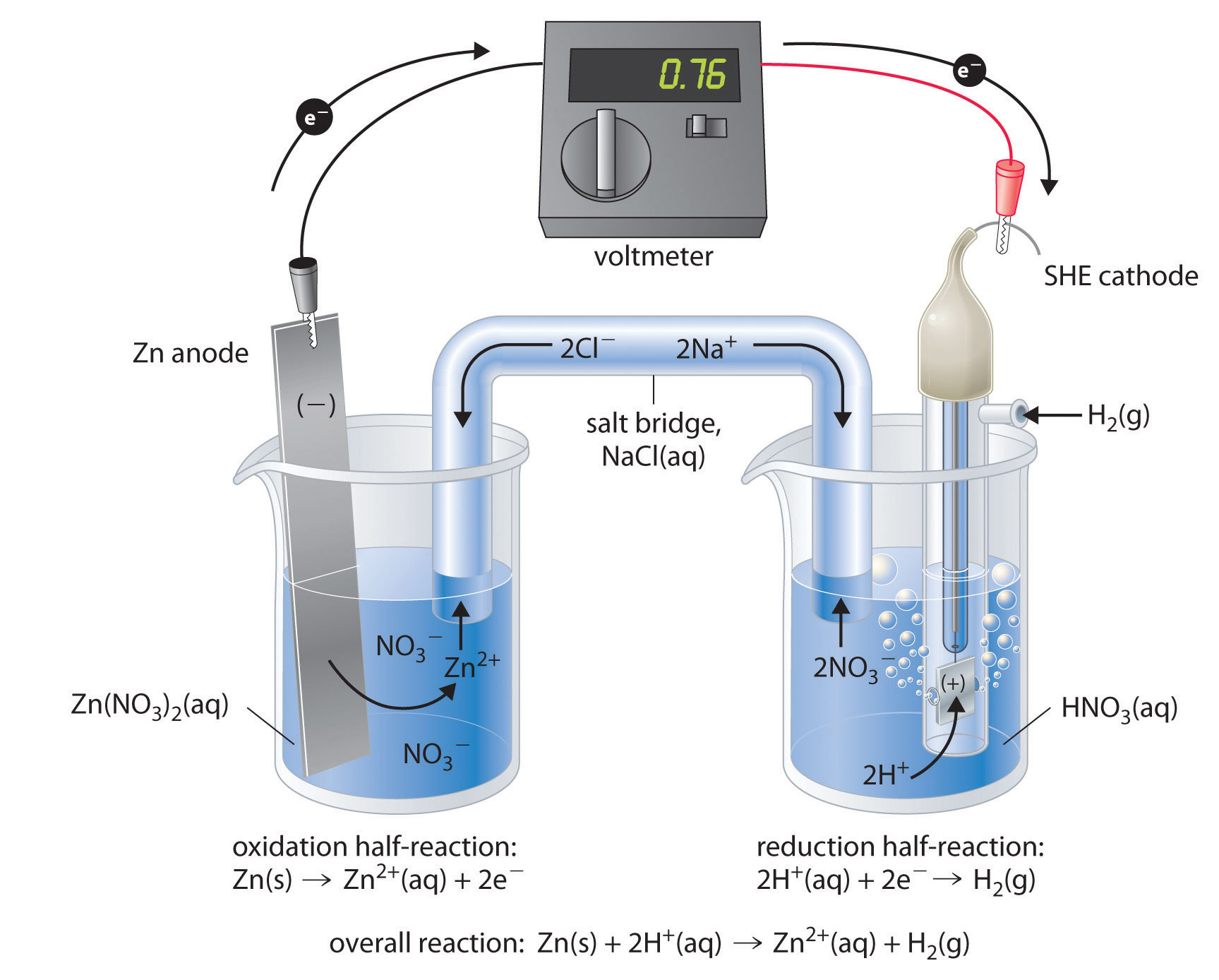
What Is The Electrochemical Used For . Electrochemical cells are divided into two basic types; Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. Voltaic cells and electrolytic cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions that move electrons is known as electrochemistry. Voltaic cells generate an electric current from. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate.
from saylordotorg.github.io
This movement of electrons is called electricity, which can be generated by movements of. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Voltaic cells and electrolytic cells. Voltaic cells generate an electric current from. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. The study of chemical reactions that move electrons is known as electrochemistry.
Electrochemistry
What Is The Electrochemical Used For This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. Voltaic cells and electrolytic cells. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The study of chemical reactions that move electrons is known as electrochemistry. This movement of electrons is called electricity, which can be generated by movements of. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells generate an electric current from. Electrochemical cells are divided into two basic types; Electricity, which is produced by the transfer of electrons from one element.
From pubs.rsc.org
Making electrochemistry easily accessible to the synthetic chemist What Is The Electrochemical Used For An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Electrochemistry is the study of chemical processes that cause electrons. What Is The Electrochemical Used For.
From scienceinfo.com
Electrochemistry Definition, Types, Components What Is The Electrochemical Used For The study of chemical reactions that move electrons is known as electrochemistry. This movement of electrons is called electricity, which can be generated by movements of. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Voltaic cells generate an electric current from. Electricity, which is produced by the transfer of. What Is The Electrochemical Used For.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Electrochemical Used For This movement of electrons is called electricity, which can be generated by movements of. Voltaic cells generate an electric current from. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry is the study of chemical processes that cause electrons to move. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely,. What Is The Electrochemical Used For.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson What Is The Electrochemical Used For Electricity, which is produced by the transfer of electrons from one element. The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. This movement of electrons is called electricity, which. What Is The Electrochemical Used For.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in What Is The Electrochemical Used For Voltaic cells generate an electric current from. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. Electrochemical cells are divided into. What Is The Electrochemical Used For.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii What Is The Electrochemical Used For Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it. What Is The Electrochemical Used For.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk What Is The Electrochemical Used For Electricity, which is produced by the transfer of electrons from one element. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it. What Is The Electrochemical Used For.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Is The Electrochemical Used For Electrochemical cells are divided into two basic types; Voltaic cells generate an electric current from. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions that move electrons is known as electrochemistry. Voltaic cells and electrolytic cells. This movement of electrons. What Is The Electrochemical Used For.
From saylordotorg.github.io
Electrochemistry What Is The Electrochemical Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This movement of electrons is called electricity, which can be generated by movements of. Electricity, which is. What Is The Electrochemical Used For.
From studylib.net
Electrochemical cells What Is The Electrochemical Used For This movement of electrons is called electricity, which can be generated by movements of. Electrochemical cells are divided into two basic types; An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Voltaic cells and electrolytic cells. Electricity, which is produced by. What Is The Electrochemical Used For.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is The Electrochemical Used For Electricity, which is produced by the transfer of electrons from one element. Voltaic cells generate an electric current from. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to. What Is The Electrochemical Used For.
From www.researchgate.net
Electrochemical vs chemical energy storage. Download Scientific Diagram What Is The Electrochemical Used For An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Electricity, which is produced by the transfer of electrons from one element. The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell splits the oxidant and reductant. What Is The Electrochemical Used For.
From www.clutchprep.com
Electrochemical Cells Analytical Chemistry Video Clutch Prep What Is The Electrochemical Used For Electricity, which is produced by the transfer of electrons from one element. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Voltaic cells and electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. What Is The Electrochemical Used For.
From www.slideshare.net
Form 4 Chapter 6 Chemistry Electrochemical Series What Is The Electrochemical Used For Voltaic cells generate an electric current from. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. The study of chemical reactions that move electrons is known as electrochemistry. Voltaic. What Is The Electrochemical Used For.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Electrochemical Used For An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. Voltaic cells generate an electric current from. This movement of electrons is called electricity, which can be generated by movements of. Electrochemical cells are divided into two basic types; An electrochemical cell is a device that can generate. What Is The Electrochemical Used For.
From saylordotorg.github.io
Electrochemistry What Is The Electrochemical Used For Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of. Electricity, which is produced by the transfer of electrons from one element. The study of chemical reactions that move electrons is known as electrochemistry. An apparatus that is used to generate electricity from a. What Is The Electrochemical Used For.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors What Is The Electrochemical Used For Voltaic cells generate an electric current from. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. This movement of electrons is called electricity, which can be generated by movements of. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is a. What Is The Electrochemical Used For.
From www.youtube.com
Electrochemical Gradients and Membrane Transport (BIOS 041) YouTube What Is The Electrochemical Used For Voltaic cells generate an electric current from. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical. What Is The Electrochemical Used For.
From saylordotorg.github.io
Electrochemistry What Is The Electrochemical Used For Voltaic cells and electrolytic cells. The study of chemical reactions that move electrons is known as electrochemistry. Electrochemical cells are divided into two basic types; This movement of electrons is called electricity, which can be generated by movements of. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is. What Is The Electrochemical Used For.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General What Is The Electrochemical Used For Electrochemistry is the study of chemical processes that cause electrons to move. Voltaic cells generate an electric current from. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. An apparatus that is used. What Is The Electrochemical Used For.
From www.collegesearch.in
Electrochemical Cell Definitions, Examples, Electrochemistry What Is The Electrochemical Used For An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Electricity, which is produced by the transfer of electrons from one element. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. What Is The Electrochemical Used For.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is The Electrochemical Used For An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of chemical processes that cause electrons to move. Voltaic cells generate an electric current from. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. What Is The Electrochemical Used For.
From www.thoughtco.com
Electrochemical Cell Definition What Is The Electrochemical Used For This movement of electrons is called electricity, which can be generated by movements of. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electricity, which is produced by the transfer of electrons from one element. Electrochemistry is the study of chemical processes that cause electrons to move. An apparatus that. What Is The Electrochemical Used For.
From www.alamy.com
Basic components of an electrochemical cell Stock Photo Alamy What Is The Electrochemical Used For An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. This movement of electrons is called electricity, which can be generated by movements of. Electricity, which is produced by the transfer of electrons from one element. Electrochemical cells are divided into two basic types; The study of chemical. What Is The Electrochemical Used For.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom What Is The Electrochemical Used For Electrochemistry is the study of chemical processes that cause electrons to move. Voltaic cells generate an electric current from. Voltaic cells and electrolytic cells. Electricity, which is produced by the transfer of electrons from one element. Electrochemical cells are divided into two basic types; Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy. What Is The Electrochemical Used For.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and What Is The Electrochemical Used For An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells and electrolytic cells. This movement of electrons is called electricity, which can be generated by movements of. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. What Is The Electrochemical Used For.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Electrochemical Used For Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. An electrochemical. What Is The Electrochemical Used For.
From 2012books.lardbucket.org
Electrochemistry What Is The Electrochemical Used For The study of chemical reactions that move electrons is known as electrochemistry. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in. What Is The Electrochemical Used For.
From mmerevise.co.uk
Electrochemical Cells MME What Is The Electrochemical Used For An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The study of chemical reactions that move electrons is known as electrochemistry. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied. What Is The Electrochemical Used For.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is The Electrochemical Used For This movement of electrons is called electricity, which can be generated by movements of. Electricity, which is produced by the transfer of electrons from one element. Voltaic cells and electrolytic cells. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. An. What Is The Electrochemical Used For.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is The Electrochemical Used For The study of chemical reactions that move electrons is known as electrochemistry. Electricity, which is produced by the transfer of electrons from one element. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Electrochemical cells are divided into two basic types;. What Is The Electrochemical Used For.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses What Is The Electrochemical Used For Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is the study of chemical processes that cause electrons to move. Electricity, which is produced by the transfer of electrons from one element. Voltaic cells and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox. What Is The Electrochemical Used For.
From www.researchgate.net
Principle of electrochemical methods (a) open circuit potential What Is The Electrochemical Used For Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. The. What Is The Electrochemical Used For.
From www.youtube.com
What is Electrochemical Cell Notation Line notation Cell Diagram What Is The Electrochemical Used For Voltaic cells generate an electric current from. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. This movement of electrons is called electricity, which can be generated by movements of. Electricity, which is produced by the transfer of electrons from one element. An apparatus that is used. What Is The Electrochemical Used For.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 What Is The Electrochemical Used For Electrochemical cells are divided into two basic types; Voltaic cells and electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the. Voltaic cells generate an electric current from. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity. What Is The Electrochemical Used For.