Area Validation Guidelines . Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. In those cases where parametric release has been. Pharmacopoeial methods should be used for the validation and performance of the sterility test.
from www.slideshare.net
Pharmacopoeial methods should be used for the validation and performance of the sterility test. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. In those cases where parametric release has been.
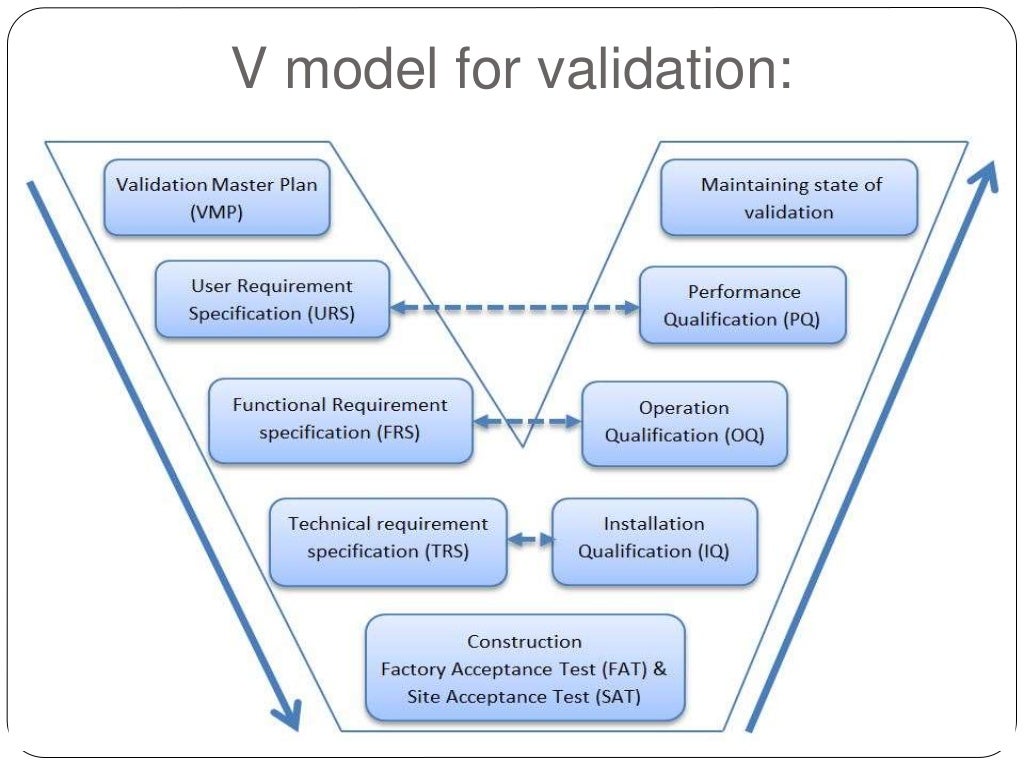
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION
Area Validation Guidelines In those cases where parametric release has been. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. In those cases where parametric release has been. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to.
From www.validation-online.net
Validation Quality Plan Responsibilities Methods Deliverables Compliance Area Validation Guidelines In those cases where parametric release has been. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal. Area Validation Guidelines.
From www.scribd.com
Process Validation Sample Protocol Verification And Validation Specification (Technical Area Validation Guidelines In those cases where parametric release has been. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. This guidance pertains to current good manufacturing practice (cgmp) regulations (21. Area Validation Guidelines.
From www.pharmaspecialists.com
Cleaning Validation in Pharmaceutical Industry Area Validation Guidelines Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Pharmacopoeial methods should be used for the validation and performance of the sterility test. In those cases where parametric release has. Area Validation Guidelines.
From www.template.net
Validation Report Templates 9+ Free Word, PDF Format Download Area Validation Guidelines This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Pharmacopoeial. Area Validation Guidelines.
From studylib.net
Guidance Validation Consultation draft Area Validation Guidelines This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. In those cases where parametric release has been. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle.. Area Validation Guidelines.
From www.presentationeze.com
Cleanroom Classification Information & current Best PracticePresentationEZE Area Validation Guidelines This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Fda. Area Validation Guidelines.
From learngxp.com
6 Key Areas for the FDA Audit for Process Validation [Video] LearnGxP Accredited Online Life Area Validation Guidelines Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Validation studies should demonstrate that. Area Validation Guidelines.
From www.slideshare.net
Analytical method validation Area Validation Guidelines Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. In those cases where. Area Validation Guidelines.
From www.pharmaceuticalonline.com
Introduction To Science And RiskBased Cleaning Validation Using ASTM E3106 E3219 Area Validation Guidelines Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This guidance pertains to. Area Validation Guidelines.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Area Validation Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. In those cases where parametric release has been. This guidance. Area Validation Guidelines.
From www.studypool.com
SOLUTION LPA guidance GMP process validation Studypool Area Validation Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Validation studies should demonstrate that class 100. Area Validation Guidelines.
From www.presentationeze.com
Aseptic Filling Process ValidationPresentationEZE Area Validation Guidelines Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This protocol entails a thorough assessment and. Area Validation Guidelines.
From www.chromatographyonline.com
Validation of StabilityIndicating HPLC Methods for Pharmaceuticals Overview, Methodologies Area Validation Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. In those cases where parametric release has been. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Fda guideline on sterile drug. Area Validation Guidelines.
From www.presentationeze.com
FDA Validation Requirements for Medical Devices PresentationEZE Area Validation Guidelines Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Pharmacopoeial methods should. Area Validation Guidelines.
From www.collidu.com
Requirement Validation PowerPoint Presentation Slides PPT Template Area Validation Guidelines This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. In those cases where parametric release has been. Pharmacopoeial methods should be used for the validation and performance of. Area Validation Guidelines.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Area Validation Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Sterile area validation has different tests like air supply, air. Area Validation Guidelines.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Area Validation Guidelines In those cases where parametric release has been. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity,. Area Validation Guidelines.
From www.slideshare.net
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION Area Validation Guidelines Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing.. Area Validation Guidelines.
From www.presentationeze.com
Equipment Validation Facility Qualification Material QualificationPresentationEZE Area Validation Guidelines This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. In those cases where parametric release has been. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. Pharmacopoeial methods should be used for the validation and performance. Area Validation Guidelines.
From www.slideshare.net
Qb d & new process validation guidance Area Validation Guidelines This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Pharmacopoeial methods should. Area Validation Guidelines.
From dokumen.tips
(PDF) 26 Site Validation of the OpenArea Test Site and the … compliance for EMI measurements Area Validation Guidelines Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Validation studies should demonstrate that. Area Validation Guidelines.
From www.scribd.com
Microbial Limit Test Validation Protocol PDF Growth Medium Microbiology Area Validation Guidelines Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. Pharmacopoeial methods should be used for the validation and performance of the sterility test. In those cases where parametric release has been. Sterile area. Area Validation Guidelines.
From www.scribd.com
Process Validation Guidance Verification And Validation Business Process Area Validation Guidelines Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. In those cases where parametric release has been. This protocol entails a thorough assessment and examination of the important. Area Validation Guidelines.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Area Validation Guidelines Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure. Area Validation Guidelines.
From www.semanticscholar.org
[PDF] Fda 2011 Process validation Guidance Process validation revisited Semantic Scholar Area Validation Guidelines This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Fda guideline on sterile drug products. Area Validation Guidelines.
From guideline-sop.com
Process Validation (PV) & Verification of Drug Product Guidelines SOPs Area Validation Guidelines Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. In those cases where parametric release has been. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation. Area Validation Guidelines.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Presentation ID6494544 Area Validation Guidelines In those cases where parametric release has been. Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies. Area Validation Guidelines.
From www.scribd.com
Validation Criteria Checklist Verification And Validation Educational Assessment Free 30 Area Validation Guidelines In those cases where parametric release has been. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This protocol entails. Area Validation Guidelines.
From www.slideserve.com
PPT The AOAC International Rapid Methods Validation Process PowerPoint Presentation ID663858 Area Validation Guidelines Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Pharmacopoeial methods should be used for the validation and performance of the sterility test. In those cases where parametric release has been. Sterile area validation has different. Area Validation Guidelines.
From www.slideserve.com
PPT Integrated Method Development and Validation PowerPoint Presentation ID5182288 Area Validation Guidelines Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. Pharmacopoeial methods should. Area Validation Guidelines.
From www.slideserve.com
PPT Process Validation What the Future Holds PowerPoint Presentation ID5665084 Area Validation Guidelines This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations This guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when manufacturing. Sterile area validation has different tests. Area Validation Guidelines.
From www.semanticscholar.org
[PDF] Fda 2011 Process validation Guidance Process validation revisited Semantic Scholar Area Validation Guidelines This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations Sterile area validation has different tests like air supply, air velocity, air changes, flow pattern, filter integrity, pressure test, particle. In those cases where. Area Validation Guidelines.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Area Validation Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. In those cases where. Area Validation Guidelines.
From www.pharmaceuticalsky.com
Analytical Method Validation Protocol for Nystatin BP Area Validation Guidelines This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Fda guideline on sterile drug products produced by aseptic processing (2004) “the goal of bacterial retention validation studies is to have. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Validation studies should demonstrate. Area Validation Guidelines.
From www.researchgate.net
(PDF) CLEANING VALIDATION IN PHARMACEUTICAL INDUSTRIES Area Validation Guidelines Validation studies should demonstrate that class 100 is maintained in critical zones during routine operations In those cases where parametric release has been. This protocol entails a thorough assessment and examination of the important zone including cleanrooms, manufacturing areas, storage spaces, and laboratories to. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This guidance. Area Validation Guidelines.