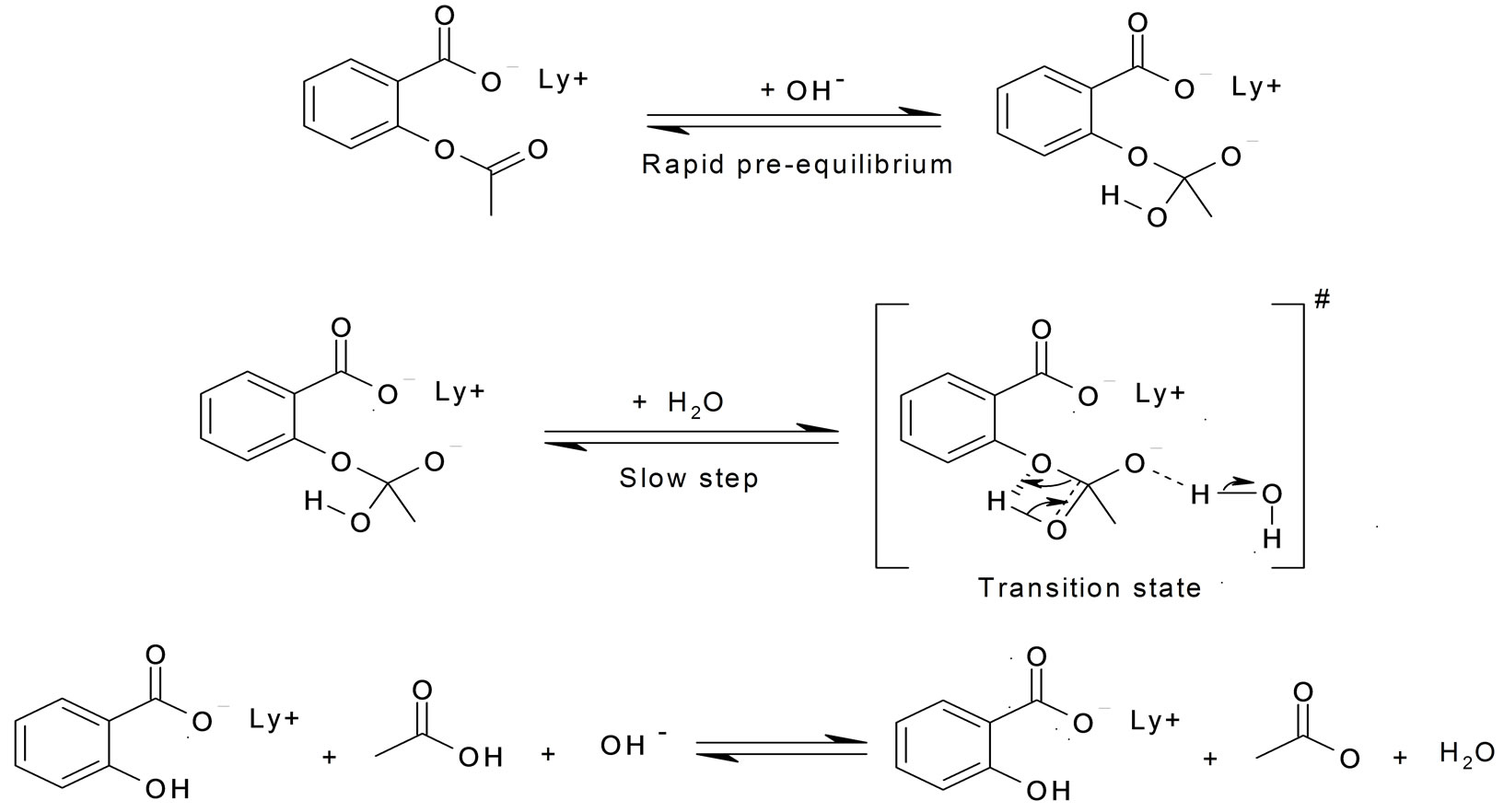
Aspirin Hydrolysis Equation . the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.
from file.scirp.org
Where a inf is the. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process.
Stability Studies of Lysine Acetylsalicylate (Aspirin Derivative
Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart.
From www.chemistrysteps.com
Acetal Hydrolysis Mechanism Chemistry Steps Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.. Aspirin Hydrolysis Equation.
From pdfprof.com
hydrolysis of aspirin mechanism and reaction Aspirin Hydrolysis Equation Where a inf is the. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.
From www.numerade.com
SOLVED 1. Write the balanced chemical formula of the reaction of Aspirin Hydrolysis Equation Where a inf is the. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.
From www.coursehero.com
[Solved] of the Hydrolysis of Aspirin. Data Analysis and Aspirin Hydrolysis Equation learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. Where a inf is the. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. the hydrolysis of the acetyl group of aspirin may occur via acid. Aspirin Hydrolysis Equation.
From chemguyv2.blogspot.com
Chemistry Experiments Aspirin Hydrolysis. Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.
From www.studocu.com
Hydrolysis of Aspirin P R A C T I C A L H Y D R O LY S I S O F A S Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.. Aspirin Hydrolysis Equation.
From bio.libretexts.org
A4. Intramolecular Catalysis Biology LibreTexts Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride. Aspirin Hydrolysis Equation.
From ar.inspiredpencil.com
Aspirin Mechanism Synthesis Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base,. Aspirin Hydrolysis Equation.
From www.numerade.com
SOLVED Write balanced reaction equations for the reactions involved Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. Where a inf is the. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.
From www.pdfprof.com
acid catalysed hydrolysis of aspirin Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.. Aspirin Hydrolysis Equation.
From www.researchgate.net
Time course of aspirin hydrolysis and LDL oxidation by the Aspirin Hydrolysis Equation Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess. Aspirin Hydrolysis Equation.
From www.youtube.com
How to Convert Aspirin to Salicylic Acid (Base Hydrolysis Method) YouTube Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.
From www.pdfprof.com
acid catalysed hydrolysis of aspirin Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.
From www.pdfprof.com
hydrolysis of aspirin lab report Aspirin Hydrolysis Equation Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base,. Aspirin Hydrolysis Equation.
From dmd.aspetjournals.org
Aspirin Hydrolysis in Human and Experimental Animal Plasma and the Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride. Aspirin Hydrolysis Equation.
From study.com
Hydrolysis of Aspirin Mechanism & Reaction Video & Lesson Transcript Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.. Aspirin Hydrolysis Equation.
From file.scirp.org
Stability Studies of Lysine Acetylsalicylate (Aspirin Derivative Aspirin Hydrolysis Equation learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most. Aspirin Hydrolysis Equation.
From mungfali.com
Solved 2. Learning How Fast The Hydrolysis Of Aspirin Occurs 406 Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed. Aspirin Hydrolysis Equation.
From www.slideserve.com
PPT UNITV DRUG STABILITY PowerPoint Presentation, free download ID Aspirin Hydrolysis Equation Where a inf is the. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base. Aspirin Hydrolysis Equation.
From file.scirp.org
Stability Studies of Lysine Acetylsalicylate (Aspirin Derivative Aspirin Hydrolysis Equation Where a inf is the. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess. Aspirin Hydrolysis Equation.
From www.researchgate.net
(PDF) Hydrolysis of aspirin. Intramolecular general base catalysis of Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.
From www.chegg.com
Solved The hydrolysis of aspirin occurs in the stomach. Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process.. Aspirin Hydrolysis Equation.
From www.pdfprof.com
hydrolysis of aspirin lab report Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis. Aspirin Hydrolysis Equation.
From www.slideserve.com
PPT From Organic Molecules to Medicines PowerPoint Presentation, free Aspirin Hydrolysis Equation Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis. Aspirin Hydrolysis Equation.
From www.reddit.com
HYDROLYSIS OF ASPIRIN r/chemistryhelp Aspirin Hydrolysis Equation Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base,. Aspirin Hydrolysis Equation.
From www.numerade.com
SOLVED Esterification reaction is used to make aspirin. In the basic Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process.. Aspirin Hydrolysis Equation.
From ibalchemy.com
D.2 Aspirin and penicillin IB Alchemy Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble. Aspirin Hydrolysis Equation.
From www.coursehero.com
[Solved] Hydrolysis of aspirin (acetylsalicylic acid). Titration of Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble. Aspirin Hydrolysis Equation.
From pdfprof.com
hydrolysis of aspirin mechanism and reaction Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis. Aspirin Hydrolysis Equation.
From talonferssampson.blogspot.com
Which of the Following Chemical Equations Describes a Hydrolysis Reaction Aspirin Hydrolysis Equation the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.. Aspirin Hydrolysis Equation.
From file.scirp.org
Stability Studies of Lysine Acetylsalicylate (Aspirin Derivative Aspirin Hydrolysis Equation learn about the hydrolysis of aspirin, whether aspirin is an acid or a base, and whether it is soluble in water. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most. Aspirin Hydrolysis Equation.
From www.jbc.org
Aspirin Hydrolysis in Plasma Is a Variable Function of Aspirin Hydrolysis Equation formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. Where a inf is the. learn about the hydrolysis of aspirin, whether aspirin is an acid or a base,. Aspirin Hydrolysis Equation.
From paperwingrvice.web.fc2.com
What is the chemical equation for the synthesis of aspirin Aspirin Hydrolysis Equation formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base catalysis, or the uncatalyzed process. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used.. Aspirin Hydrolysis Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation that corresponds to the Aspirin Hydrolysis Equation we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. Where a inf is the. the hydrolysis of the acetyl group of aspirin may occur via acid catalysis, base. Aspirin Hydrolysis Equation.
From chemguyv2.blogspot.com
Chemistry Experiments Aspirin Hydrolysis. Aspirin Hydrolysis Equation Where a inf is the. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin, probably the most commonly used. formation of acetylsalicylic acid (asa, a.k.a., aspirin) at this point the excess acetic anhydride must be hydrolyzed (split apart. we can use equation \(\ref{eq1}\) to determine the reaction rate of hydrolysis of aspirin,. Aspirin Hydrolysis Equation.