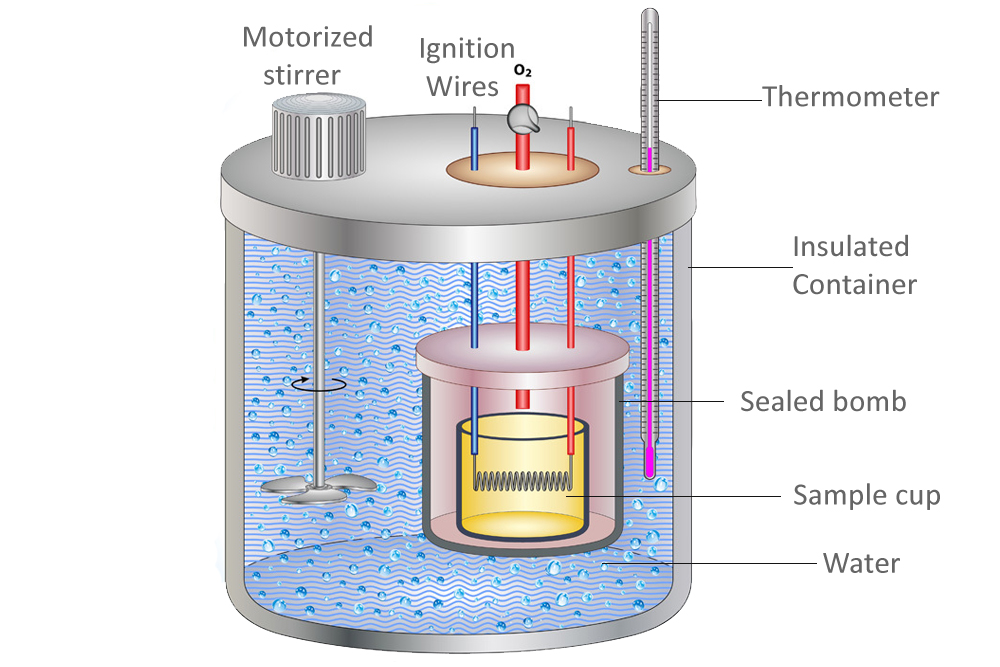
Calorimeter Have Heat Capacity . compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Discern the difference between constant. Calculate and interpret heat and related properties using typical. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction.
from www.scienceabc.com
Explain the technique of calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Calculate heat, temperature change, and specific heat after. Discern the difference between constant. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate and interpret heat and related properties using typical. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction.
Molar Heat Capacity Definition, Formula, Equation, Calculation
Calorimeter Have Heat Capacity Calculate and interpret heat and related properties using typical. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Calculate and interpret heat and related properties using typical. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calculate heat, temperature change, and specific heat after. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. Discern the difference between constant. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Explain the technique of calorimetry. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Have Heat Capacity in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. compare heat flow from hot to cold objects in. Calorimeter Have Heat Capacity.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Calorimeter Have Heat Capacity the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. Calculate and interpret heat and related properties using typical. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον. Calorimeter Have Heat Capacity.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Have Heat Capacity Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. Discern the difference between constant. Calculate heat, temperature change, and specific heat after. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. compare heat flow from hot to cold objects in an ideal calorimeter. Calorimeter Have Heat Capacity.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Calorimeter Have Heat Capacity in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. In this article, you will learn about calorimetry. Calorimeter Have Heat Capacity.
From www.youtube.com
G10 Chemistry Calorimetry and Specific Heat Capacity Worked Examples Calorimeter Have Heat Capacity Discern the difference between constant. Explain the technique of calorimetry. Calculate heat, temperature change, and specific heat after. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calculate and interpret heat and related properties using typical. compare heat flow from hot to cold objects in an ideal calorimeter versus. Calorimeter Have Heat Capacity.
From www.studypool.com
SOLUTION Heat Capacity Of Calorimeter Studypool Calorimeter Have Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. in chemistry and thermodynamics, calorimetry (from latin. Calorimeter Have Heat Capacity.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Have Heat Capacity the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Discern the difference between constant. Calculate and interpret heat and related properties using typical. detail how calorimetry can be used to determine. Calorimeter Have Heat Capacity.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Calorimeter Have Heat Capacity Calculate heat, temperature change, and specific heat after. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. Discern the difference between constant. In this article, you will learn about calorimetry and calorimeters, mainly used. Calorimeter Have Heat Capacity.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep Calorimeter Have Heat Capacity Discern the difference between constant. Calculate and interpret heat and related properties using typical. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Explain the technique of calorimetry. Calculate heat, temperature change, and specific heat after. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον. Calorimeter Have Heat Capacity.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Have Heat Capacity Discern the difference between constant. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Explain the technique of calorimetry. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after. detail how calorimetry can be used to. Calorimeter Have Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Have Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Explain the technique of. Calorimeter Have Heat Capacity.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimeter Have Heat Capacity Calculate and interpret heat and related properties using typical. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure'). Calorimeter Have Heat Capacity.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog Calorimeter Have Heat Capacity Explain the technique of calorimetry. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Discern the difference between constant. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. the magnitude of the temperature change depends on both the amount of thermal. Calorimeter Have Heat Capacity.
From slidetodoc.com
CHEM 1011 Calorimetry The Determination of the Specific Calorimeter Have Heat Capacity detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. In this article,. Calorimeter Have Heat Capacity.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimeter Have Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. in chemistry and thermodynamics, calorimetry (from. Calorimeter Have Heat Capacity.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Have Heat Capacity Calculate and interpret heat and related properties using typical. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. Explain the technique of calorimetry. Discern the difference between constant. compare heat flow. Calorimeter Have Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Have Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. Discern the difference between constant. Explain the technique of calorimetry. the magnitude of the temperature change depends on both the amount of. Calorimeter Have Heat Capacity.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimeter Have Heat Capacity Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. the magnitude of the temperature change depends on both the amount of. Calorimeter Have Heat Capacity.
From www.slideserve.com
PPT CHEM 1011 PowerPoint Presentation, free download ID317228 Calorimeter Have Heat Capacity Explain the technique of calorimetry. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. the. Calorimeter Have Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Have Heat Capacity Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry. Discern the difference between constant. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. detail. Calorimeter Have Heat Capacity.
From www.slideserve.com
PPT CHEM 1011 PowerPoint Presentation, free download ID317228 Calorimeter Have Heat Capacity Explain the technique of calorimetry. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Discern the difference between constant. Calculate and interpret heat and related properties using typical. compare heat flow from hot. Calorimeter Have Heat Capacity.
From studylib.net
Chapter 5b (PowerPoint) Calorimeter Have Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Explain the technique of. Calorimeter Have Heat Capacity.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Have Heat Capacity the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Explain the technique of calorimetry. Calculate heat, temperature change, and specific heat after. detail how calorimetry can be used to determine the. Calorimeter Have Heat Capacity.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Have Heat Capacity Explain the technique of calorimetry. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Calculate heat, temperature change, and specific heat after. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Discern the difference between constant. detail how calorimetry can. Calorimeter Have Heat Capacity.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Have Heat Capacity Explain the technique of calorimetry. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is. Calorimeter Have Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Calorimeter Have Heat Capacity compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate and interpret heat and related properties using typical. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. detail. Calorimeter Have Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Have Heat Capacity In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calculate heat, temperature change, and specific heat after. detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. the magnitude of the temperature change depends on both the amount of thermal energy. Calorimeter Have Heat Capacity.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Have Heat Capacity Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Discern the difference between constant. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. detail how calorimetry can be used to. Calorimeter Have Heat Capacity.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Have Heat Capacity Calculate heat, temperature change, and specific heat after. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Discern the difference between constant. In this article, you will learn about calorimetry and calorimeters, mainly used to. Calorimeter Have Heat Capacity.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter Have Heat Capacity Calculate and interpret heat and related properties using typical. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. compare. Calorimeter Have Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Have Heat Capacity in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calculate and interpret heat and related properties using typical. Calculate heat, temperature change, and specific heat after. compare heat flow from hot to. Calorimeter Have Heat Capacity.
From slideplayer.com
Specific Heat Capacity & Calorimetry ppt download Calorimeter Have Heat Capacity Calculate heat, temperature change, and specific heat after. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Discern the difference between constant. in chemistry and thermodynamics, calorimetry (from latin calor 'heat'. Calorimeter Have Heat Capacity.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Have Heat Capacity Explain the technique of calorimetry. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Calculate. Calorimeter Have Heat Capacity.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Calorimeter Have Heat Capacity detail how calorimetry can be used to determine the amount of energy released in a chemical reaction. Discern the difference between constant. Explain the technique of calorimetry. compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after. Calculate and interpret heat and related properties. Calorimeter Have Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Have Heat Capacity in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. the magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat. Calculate heat, temperature change, and specific heat after. Explain the technique of calorimetry. detail how calorimetry can be used to determine the. Calorimeter Have Heat Capacity.