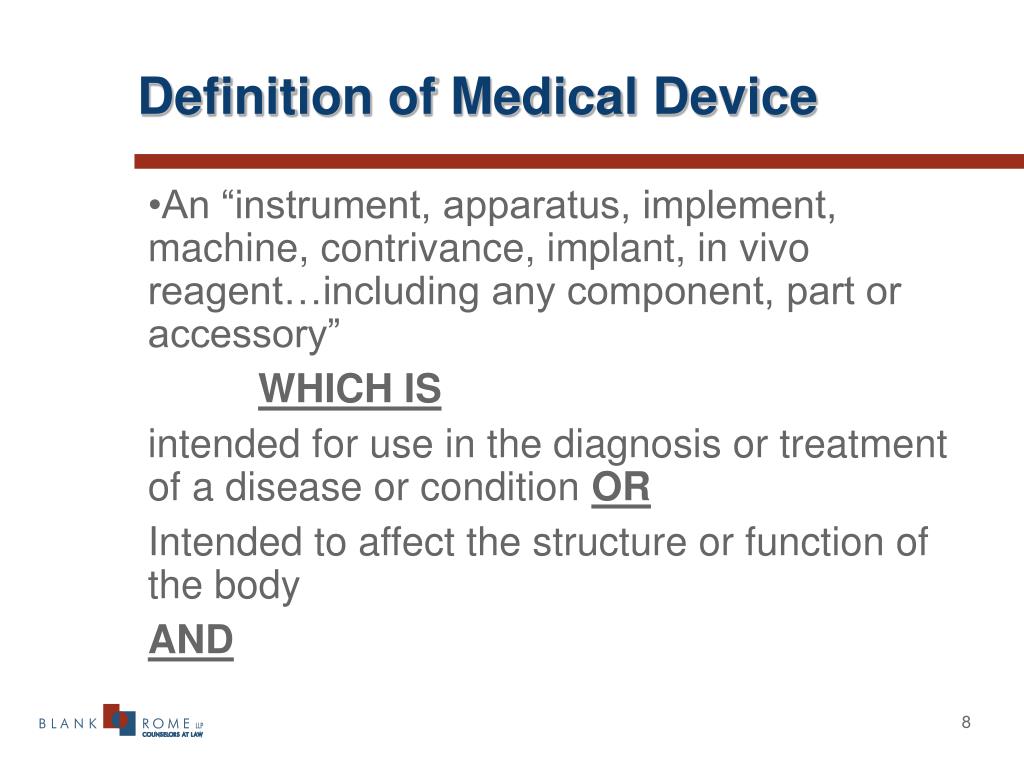
Medical Device Definition Who . 1.2 limitations of the who. It can be used either alone or in combination. (1) ‘medical device’ means any instrument,. The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. 1.1 the who global model regulatory framework for medical devices including ivds. For the purposes of this regulation, the following definitions apply: Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; We propose the following definition of a medical device: The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. “a contrivance designed and manufactured for use in healthcare, and not.
from www.slideserve.com
“a contrivance designed and manufactured for use in healthcare, and not. We propose the following definition of a medical device: Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; For the purposes of this regulation, the following definitions apply: 1.2 limitations of the who. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. (1) ‘medical device’ means any instrument,. It can be used either alone or in combination. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical.
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation
Medical Device Definition Who We propose the following definition of a medical device: The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. 1.2 limitations of the who. The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. It can be used either alone or in combination. 1.1 the who global model regulatory framework for medical devices including ivds. We propose the following definition of a medical device: Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; For the purposes of this regulation, the following definitions apply: “a contrivance designed and manufactured for use in healthcare, and not. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. (1) ‘medical device’ means any instrument,.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Definition Who A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. We propose the following definition of a medical device: It can be used either alone or in combination. For the purposes of this regulation, the. Medical Device Definition Who.
From cliniexperts.com
ISO 13485 Medical Devices Certification Medical Device ISO Standards Medical Device Definition Who We propose the following definition of a medical device: “a contrivance designed and manufactured for use in healthcare, and not. 1.1 the who global model regulatory framework for medical devices including ivds. 1.2 limitations of the who. For the purposes of this regulation, the following definitions apply: The who medical device technical series consists of reference documents to assist countries. Medical Device Definition Who.
From waddellgrp.com
So you want to make a Medical Device . . . Medical Project Management Medical Device Definition Who 1.1 the who global model regulatory framework for medical devices including ivds. (1) ‘medical device’ means any instrument,. “a contrivance designed and manufactured for use in healthcare, and not. It can be used either alone or in combination. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; A medical. Medical Device Definition Who.
From www.pinterest.com
What is a Medical Device? (Official definition for EU, USA, China Medical Device Definition Who 1.2 limitations of the who. “a contrivance designed and manufactured for use in healthcare, and not. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. The who medical device technical series consists. Medical Device Definition Who.
From unifymedsupply.com
Difference between Medical Devices and Medical Consumables Medical Device Definition Who Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. “a contrivance designed and manufactured for use in healthcare, and not. It can be used either alone or in combination. (1) ‘medical. Medical Device Definition Who.
From www.arenasolutions.com
Medical Device Definition Arena Medical Device Definition Who (1) ‘medical device’ means any instrument,. “a contrivance designed and manufactured for use in healthcare, and not. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. The who medical device technical series. Medical Device Definition Who.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Definition Who The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. 1.1 the who global model regulatory framework for medical devices including ivds. 1.2 limitations of the who. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. It can be. Medical Device Definition Who.
From fyoqglxgv.blob.core.windows.net
What Is A Medical Device Fda Definition at Theresa Ward blog Medical Device Definition Who 1.1 the who global model regulatory framework for medical devices including ivds. For the purposes of this regulation, the following definitions apply: Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality.. Medical Device Definition Who.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Medical Device Definition Who A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; It can be used either alone or in combination. (1) ‘medical device’ means any instrument,. “a contrivance designed and manufactured for use in. Medical Device Definition Who.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Definition Who For the purposes of this regulation, the following definitions apply: 1.2 limitations of the who. “a contrivance designed and manufactured for use in healthcare, and not. We propose the following definition of a medical device: The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. (1) ‘medical. Medical Device Definition Who.
From kantify.com
Kantify Improving Human Health through Artificial Intelligence Medical Device Definition Who It can be used either alone or in combination. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. “a contrivance designed and manufactured for use in healthcare, and not. 1.1 the who global model regulatory framework for medical devices including ivds. A medical device can be any instrument, apparatus, implement, machine,. Medical Device Definition Who.
From candlemakingclasseshoustontxyamachifu.blogspot.com
Candle Making Classes Houston Tx Fda Class 2 Medical Device Definition Medical Device Definition Who “a contrivance designed and manufactured for use in healthcare, and not. 1.2 limitations of the who. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. We. Medical Device Definition Who.
From market.us
Medical Device Industry Statistics, Fact, Growth Market.us Medical Device Definition Who 1.2 limitations of the who. It can be used either alone or in combination. The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. For the purposes of this regulation, the following definitions apply: “a contrivance designed and manufactured for use in healthcare, and not. Medical equipment. Medical Device Definition Who.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Medical Device Definition Who We propose the following definition of a medical device: (1) ‘medical device’ means any instrument,. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. For the purposes of this regulation, the following definitions apply: It can be used either alone or in combination. The who medical device technical series consists of reference. Medical Device Definition Who.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Device Definition Who The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. For the purposes of this regulation, the following definitions apply: It can be used either alone or in combination. “a contrivance designed and manufactured for use in healthcare, and not. The who medical device technical series consists. Medical Device Definition Who.
From veraqueconsulting.com
The Medical Device Manufacturer definition (The Mexico case) Veraque Medical Device Definition Who 1.2 limitations of the who. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. We propose the following definition of a medical device: A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. (1) ‘medical device’ means any instrument,. It can be used either. Medical Device Definition Who.
From sonkahealth.com
According to the MDR, what kind of equipment is considered a medical Medical Device Definition Who It can be used either alone or in combination. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; We propose the following definition of a medical device: (1) ‘medical device’ means any. Medical Device Definition Who.
From reviewmotors.co
Medical Device Directive Spare Parts List Reviewmotors.co Medical Device Definition Who A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. “a contrivance designed and manufactured for use in healthcare, and not. Medical equipment is used for the specific purposes of diagnosis and treatment of disease. Medical Device Definition Who.
From operonstrategist.com
Software as a Medical Device (SaMD) IEC 62304 Certification Medical Device Definition Who “a contrivance designed and manufactured for use in healthcare, and not. We propose the following definition of a medical device: 1.2 limitations of the who. 1.1 the who global model regulatory framework for medical devices including ivds. It can be used either alone or in combination. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for. Medical Device Definition Who.
From medicaldevicehq.com
What is a medical device according to the MDR Medical Device HQ 1 Medical Device Definition Who “a contrivance designed and manufactured for use in healthcare, and not. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. For the purposes of this regulation, the following definitions apply: We propose the following definition of a medical device: A medical device can be any instrument, apparatus, implement, machine, appliance, implant,. Medical Device Definition Who.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 Medical Device Definition Who The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. 1.2 limitations of the who. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument,. We propose the following. Medical Device Definition Who.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Device Definition Who We propose the following definition of a medical device: For the purposes of this regulation, the following definitions apply: It can be used either alone or in combination. 1.2 limitations of the who. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. “a contrivance designed and manufactured for use in healthcare,. Medical Device Definition Who.
From news.iscas.co
Interoperability standards for medical device integration in the OR and Medical Device Definition Who It can be used either alone or in combination. “a contrivance designed and manufactured for use in healthcare, and not. For the purposes of this regulation, the following definitions apply: The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. A medical device can be any instrument,. Medical Device Definition Who.
From candlemakingclasseshoustontxyamachifu.blogspot.com
Candle Making Classes Houston Tx Fda Class 2 Medical Device Definition Medical Device Definition Who (1) ‘medical device’ means any instrument,. We propose the following definition of a medical device: For the purposes of this regulation, the following definitions apply: It can be used either alone or in combination. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical equipment is used for the specific purposes of. Medical Device Definition Who.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Definition Who The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. It can. Medical Device Definition Who.
From medicaldevicehq.com
What is a medical device? Medical Device HQ Medical Device Definition Who We propose the following definition of a medical device: The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. (1) ‘medical device’ means any instrument,. For the purposes of this regulation, the following definitions apply: Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following. Medical Device Definition Who.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations Medical Device Definition Who 1.2 limitations of the who. The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. We propose the following definition of a medical device: The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. (1) ‘medical device’ means any. Medical Device Definition Who.
From blog.pharmadra.net
Medical deviceDefinition Pharma DRA Medical Device Definition Who The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or. Medical Device Definition Who.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Definition Who The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. It can be used either alone or in combination. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; For the purposes of this regulation, the following. Medical Device Definition Who.
From de.slideshare.net
Medical device definition Medical Device Definition Who The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury;. Medical Device Definition Who.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Device Definition Who We propose the following definition of a medical device: Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; “a contrivance designed and manufactured for use in healthcare, and not. It can be used either alone or in combination. The who therefore convened the priority medical devices (pmd) project to. Medical Device Definition Who.
From www.youtube.com
What are medical devices? YouTube Medical Device Definition Who 1.1 the who global model regulatory framework for medical devices including ivds. It can be used either alone or in combination. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; The who medical device technical series consists of reference documents to assist countries in ensuring improved access, quality. The. Medical Device Definition Who.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Medical Device Definition Who (1) ‘medical device’ means any instrument,. It can be used either alone or in combination. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. We propose the following definition of a medical device: The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to. Medical Device Definition Who.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Definition Who (1) ‘medical device’ means any instrument,. “a contrivance designed and manufactured for use in healthcare, and not. We propose the following definition of a medical device: 1.2 limitations of the who. It can be used either alone or in combination. The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve. Medical Device Definition Who.
From www.qualitymeddev.com
Medical Device Definition according to EU MDR 2017/745 Medical Device Definition Who The who therefore convened the priority medical devices (pmd) project to emphasize the need for a global agenda to improve access to medical. For the purposes of this regulation, the following definitions apply: It can be used either alone or in combination. We propose the following definition of a medical device: 1.2 limitations of the who. “a contrivance designed and. Medical Device Definition Who.