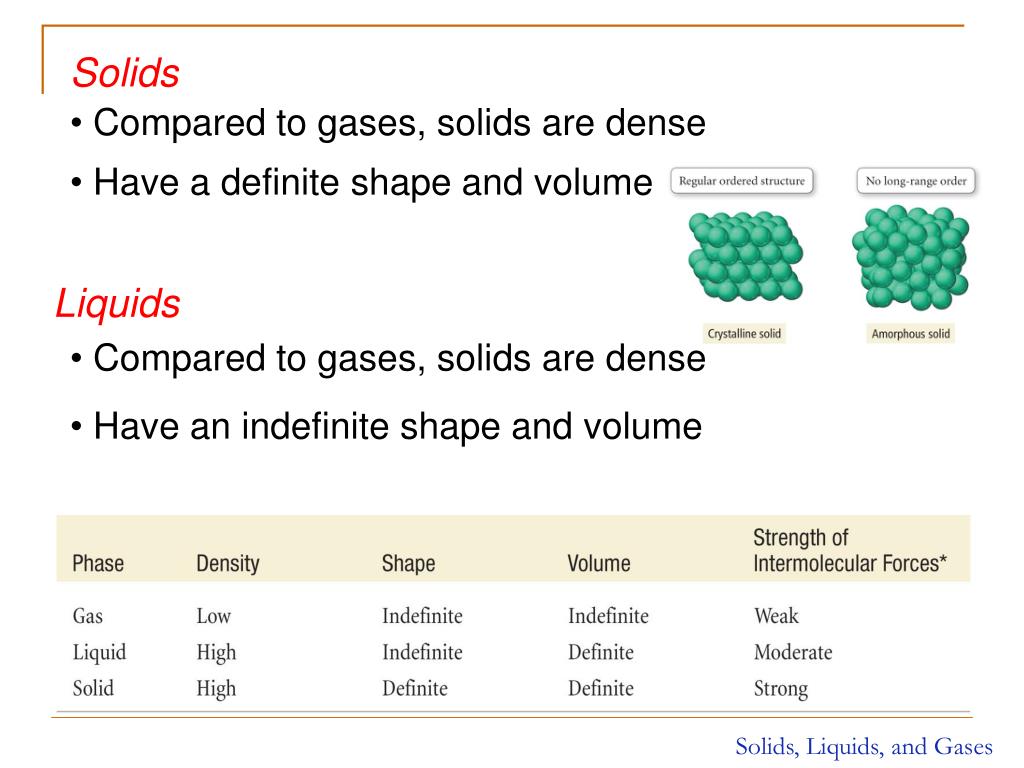
Solids Liquids And Gases Intermolecular Forces . In general covalent bonds determine: The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Solids with highly ordered structures are said to be crystalline. The equilibrium between a liquid and its vapor depends on the temperature of the system; Converting a gas into a liquid or. These are forces between molecules. Solids and liquids are condensed phases. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. All liquids experience capillary action,. Physical properties of liquids and solids are due to intermolecular forces.
from www.slideserve.com
All liquids experience capillary action,. In general covalent bonds determine: The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. These are forces between molecules. Solids with highly ordered structures are said to be crystalline. Physical properties of liquids and solids are due to intermolecular forces. Solids and liquids are condensed phases. Converting a gas into a liquid or. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of the system;
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces
Solids Liquids And Gases Intermolecular Forces These are forces between molecules. Solids with highly ordered structures are said to be crystalline. Solids and liquids are condensed phases. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Converting a gas into a liquid or. All liquids experience capillary action,. Physical properties of liquids and solids are due to intermolecular forces. These are forces between molecules. The equilibrium between a liquid and its vapor depends on the temperature of the system; In general covalent bonds determine: All liquids experience surface tension, an imbalance of forces at the surface of the liquid.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Solids Liquids And Gases Intermolecular Forces Solids and liquids are condensed phases. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Solids with highly ordered structures are said to be crystalline. The equilibrium between a liquid and its vapor depends on the temperature of the system; These are forces between. Solids Liquids And Gases Intermolecular Forces.
From www.snexplores.org
Explainer What are the different states of matter? Solids Liquids And Gases Intermolecular Forces All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Converting a gas into a liquid or. The equilibrium between a liquid and its vapor depends on the temperature of the system; Physical properties of liquids and solids are due to intermolecular forces. All liquids experience capillary action,. Solids and liquids are condensed phases. The. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces Solids Liquids And Gases Intermolecular Forces Physical properties of liquids and solids are due to intermolecular forces. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Solids with highly ordered structures are said to be crystalline. In general covalent bonds determine: These are forces between molecules. Solids and liquids are. Solids Liquids And Gases Intermolecular Forces.
From www.nagwa.com
Question Video Representing How Intermolecular Forces Change as Solids Liquids And Gases Intermolecular Forces The equilibrium between a liquid and its vapor depends on the temperature of the system; Solids with highly ordered structures are said to be crystalline. These are forces between molecules. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Solids and liquids are condensed phases. All liquids experience capillary action,. In general covalent bonds. Solids Liquids And Gases Intermolecular Forces.
From www.meritnation.com
Give the differences between solids, liquids and gases on the basis of Solids Liquids And Gases Intermolecular Forces All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of the system; Solids and liquids are condensed phases. Solids with highly ordered structures are said to be crystalline. In general covalent bonds determine: Physical properties of liquids and solids are due to. Solids Liquids And Gases Intermolecular Forces.
From www.ck12.org
Intermolecular Forces of Attraction CK12 Foundation Solids Liquids And Gases Intermolecular Forces Converting a gas into a liquid or. These are forces between molecules. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Solids with highly ordered structures are said to be crystalline. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or. Solids Liquids And Gases Intermolecular Forces.
From www.askiitians.com
General Characteristics of Solid State Study Material for IIT JEE Solids Liquids And Gases Intermolecular Forces The equilibrium between a liquid and its vapor depends on the temperature of the system; In general covalent bonds determine: All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Converting a gas into a liquid or. Physical properties of liquids and solids are due to intermolecular forces. These are forces between molecules. The differences. Solids Liquids And Gases Intermolecular Forces.
From sherryleeede.blogspot.com
diagram of particles in a solid SherryleeEde Solids Liquids And Gases Intermolecular Forces Converting a gas into a liquid or. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Physical properties of liquids and solids are due to intermolecular forces. Solids and liquids are condensed phases. All liquids experience surface tension, an imbalance of forces at the. Solids Liquids And Gases Intermolecular Forces.
From www.youtube.com
Intermolecular Forces of Liquids and Solids YouTube Solids Liquids And Gases Intermolecular Forces In general covalent bonds determine: The equilibrium between a liquid and its vapor depends on the temperature of the system; The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Solids with highly ordered structures are said to be crystalline. Converting a gas into a. Solids Liquids And Gases Intermolecular Forces.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.1.1 The Three States of Matter翰林国际教育 Solids Liquids And Gases Intermolecular Forces In general covalent bonds determine: These are forces between molecules. Solids with highly ordered structures are said to be crystalline. All liquids experience capillary action,. Solids and liquids are condensed phases. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of the. Solids Liquids And Gases Intermolecular Forces.
From www.slideshare.net
Intermolecular forces (liquids and solids) Solids Liquids And Gases Intermolecular Forces Solids and liquids are condensed phases. All liquids experience capillary action,. These are forces between molecules. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Physical properties of liquids and solids are due to intermolecular forces. The equilibrium between a liquid and its vapor. Solids Liquids And Gases Intermolecular Forces.
From www.gasrebate.net
Properties Of Solids Liquids Gases Compared Teachoo Science Solids Liquids And Gases Intermolecular Forces The equilibrium between a liquid and its vapor depends on the temperature of the system; The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. These are forces between molecules. In general covalent bonds determine: Solids and liquids are condensed phases. All liquids experience surface. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Solids Liquids And Gases Intermolecular Forces Solids with highly ordered structures are said to be crystalline. Solids and liquids are condensed phases. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of the system; Converting a gas into a liquid or. Physical properties of liquids and solids are. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids Solids Liquids And Gases Intermolecular Forces All liquids experience capillary action,. The equilibrium between a liquid and its vapor depends on the temperature of the system; These are forces between molecules. Physical properties of liquids and solids are due to intermolecular forces. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions. Solids Liquids And Gases Intermolecular Forces.
From www.ecolebooks.com
PHYSICS O LEVEL(FORM ONE) TOPIC 6 STRUCTURE AND PROPERTIES OF MATTER Solids Liquids And Gases Intermolecular Forces Converting a gas into a liquid or. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. The equilibrium between a liquid and its vapor depends on the temperature of the system; In general covalent bonds determine: Solids with highly ordered structures are said to. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Solids Liquids And Gases Intermolecular Forces In general covalent bonds determine: Converting a gas into a liquid or. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. These are forces between molecules. Solids with highly ordered structures are said to be crystalline. All liquids experience capillary action,. The differences in the properties of a solid, liquid, or gas reflect the. Solids Liquids And Gases Intermolecular Forces.
From jennybmooreo.blob.core.windows.net
Solid Liquid Gas Solution at jennybmooreo blog Solids Liquids And Gases Intermolecular Forces Physical properties of liquids and solids are due to intermolecular forces. In general covalent bonds determine: Converting a gas into a liquid or. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. All liquids experience capillary action,. Solids with highly ordered structures are said. Solids Liquids And Gases Intermolecular Forces.
From diagramlibraryconjoin.z19.web.core.windows.net
Solid Liquid And Gas Diagram Solids Liquids And Gases Intermolecular Forces Solids and liquids are condensed phases. The equilibrium between a liquid and its vapor depends on the temperature of the system; The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. In general covalent bonds determine: Solids with highly ordered structures are said to be. Solids Liquids And Gases Intermolecular Forces.
From documents.pub
Intermolecular Forces Important differences between gases, solids Solids Liquids And Gases Intermolecular Forces Solids and liquids are condensed phases. All liquids experience capillary action,. Solids with highly ordered structures are said to be crystalline. Physical properties of liquids and solids are due to intermolecular forces. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Solids Liquids And Gases Intermolecular Forces Solids with highly ordered structures are said to be crystalline. The equilibrium between a liquid and its vapor depends on the temperature of the system; Solids and liquids are condensed phases. In general covalent bonds determine: Physical properties of liquids and solids are due to intermolecular forces. These are forces between molecules. All liquids experience surface tension, an imbalance of. Solids Liquids And Gases Intermolecular Forces.
From byjus.com
Which of the following shows Highest interpartical spaces Highest Solids Liquids And Gases Intermolecular Forces Converting a gas into a liquid or. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of the system; Solids with highly ordered structures are said to be crystalline. The differences in the properties of a solid, liquid, or gas reflect the. Solids Liquids And Gases Intermolecular Forces.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector Solids Liquids And Gases Intermolecular Forces All liquids experience capillary action,. In general covalent bonds determine: All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Solids with highly ordered structures are said to be crystalline. Converting a gas into a liquid or. These are forces between molecules. The differences in the properties of a solid, liquid, or gas reflect the. Solids Liquids And Gases Intermolecular Forces.
From anahiadddougherty.blogspot.com
Gas to Liquid is Called AnahiaddDougherty Solids Liquids And Gases Intermolecular Forces All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of the system; Converting a gas into a liquid or. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or. Solids Liquids And Gases Intermolecular Forces.
From philschatz.com
Intermolecular Forces · Chemistry Solids Liquids And Gases Intermolecular Forces Solids and liquids are condensed phases. These are forces between molecules. Converting a gas into a liquid or. Solids with highly ordered structures are said to be crystalline. Physical properties of liquids and solids are due to intermolecular forces. All liquids experience capillary action,. The equilibrium between a liquid and its vapor depends on the temperature of the system; All. Solids Liquids And Gases Intermolecular Forces.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solids Liquids And Gases Intermolecular Forces All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Solids with highly ordered structures are said to be crystalline. The equilibrium between a liquid and its vapor depends on the temperature of the system; These are forces between molecules. Converting a gas into a liquid or. Solids and liquids are condensed phases. All liquids. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint Solids Liquids And Gases Intermolecular Forces In general covalent bonds determine: Physical properties of liquids and solids are due to intermolecular forces. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Converting a gas into a liquid or. The equilibrium between a liquid and its vapor depends on the temperature of the system; The differences in the properties of a. Solids Liquids And Gases Intermolecular Forces.
From www.studypool.com
SOLUTION Gases intermolecular forces liquids and solids Studypool Solids Liquids And Gases Intermolecular Forces The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. All liquids experience capillary action,. Physical properties of liquids and solids are due to intermolecular forces. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. These are forces between. Solids Liquids And Gases Intermolecular Forces.
From dokumen.tips
(PPT) Condensed Phases and Intermolecular Forces. Fundamentals How do Solids Liquids And Gases Intermolecular Forces Solids and liquids are condensed phases. All liquids experience capillary action,. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Solids with highly ordered structures are said to be crystalline. In general covalent bonds determine: All liquids experience surface tension, an imbalance of forces. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation Solids Liquids And Gases Intermolecular Forces The equilibrium between a liquid and its vapor depends on the temperature of the system; Physical properties of liquids and solids are due to intermolecular forces. All liquids experience capillary action,. These are forces between molecules. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The differences in the properties of a solid, liquid,. Solids Liquids And Gases Intermolecular Forces.
From www.youtube.com
Introduction to solids, liquids, and intermolecular forces YouTube Solids Liquids And Gases Intermolecular Forces All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Physical properties of liquids and solids are due to intermolecular forces. These are forces between molecules. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Solids with highly ordered. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Solids Liquids And Gases Intermolecular Forces All liquids experience capillary action,. In general covalent bonds determine: The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Converting a gas into a liquid or. Solids and liquids are condensed phases. The equilibrium between a liquid and its vapor depends on the temperature. Solids Liquids And Gases Intermolecular Forces.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solids Liquids And Gases Intermolecular Forces All liquids experience surface tension, an imbalance of forces at the surface of the liquid. The equilibrium between a liquid and its vapor depends on the temperature of the system; All liquids experience capillary action,. These are forces between molecules. Physical properties of liquids and solids are due to intermolecular forces. In general covalent bonds determine: The differences in the. Solids Liquids And Gases Intermolecular Forces.
From schoolbag.info
For a given substance, do you expect the density of the substance in Solids Liquids And Gases Intermolecular Forces In general covalent bonds determine: Converting a gas into a liquid or. Solids with highly ordered structures are said to be crystalline. All liquids experience capillary action,. Solids and liquids are condensed phases. Physical properties of liquids and solids are due to intermolecular forces. The differences in the properties of a solid, liquid, or gas reflect the strengths of the. Solids Liquids And Gases Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Solids Liquids And Gases Intermolecular Forces The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that. Physical properties of liquids and solids are due to intermolecular forces. The equilibrium between a liquid and its vapor depends on the temperature of the system; All liquids experience surface tension, an imbalance of forces. Solids Liquids And Gases Intermolecular Forces.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix Solids Liquids And Gases Intermolecular Forces All liquids experience capillary action,. The equilibrium between a liquid and its vapor depends on the temperature of the system; Solids with highly ordered structures are said to be crystalline. All liquids experience surface tension, an imbalance of forces at the surface of the liquid. Converting a gas into a liquid or. Physical properties of liquids and solids are due. Solids Liquids And Gases Intermolecular Forces.